当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Novel Pyrazole-Quinazoline-2,4-dione Hybrids as 4-Hydroxyphenylpyruvate Dioxygenase Inhibitors.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-04-22 , DOI: 10.1021/acs.jafc.0c00051 Bo He 1 , Feng-Xu Wu 1 , Liang-Kun Yu 1 , Lei Wu 1 , Qiong Chen 1 , Ge-Fei Hao 1 , Wen-Chao Yang 1 , Hong-Yan Lin 1 , Guang-Fu Yang 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-04-22 , DOI: 10.1021/acs.jafc.0c00051 Bo He 1 , Feng-Xu Wu 1 , Liang-Kun Yu 1 , Lei Wu 1 , Qiong Chen 1 , Ge-Fei Hao 1 , Wen-Chao Yang 1 , Hong-Yan Lin 1 , Guang-Fu Yang 1, 2
Affiliation
|
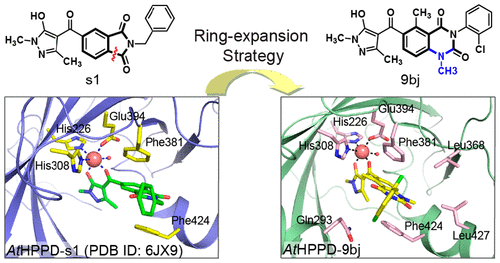
4-Hydroxyphenylpyruvate dioxygenase (HPPD, EC 1.13.11.27) has been identified as one of the most significant targets in herbicide discovery for resistant weed control. In a continuing effort to discover potent novel HPPD inhibitors, we adopted a ring-expansion strategy to design a series of novel pyrazole-quinazoline-2,4-dione hybrids based on the previously discovered pyrazole-isoindoline-1,3-dione scaffold. One compound, 3-(2-chlorophenyl)-6-(5-hydroxy-1,3-dimethyl-1H-pyrazole-4-carbonyl)-1,5-dimethylquinazoline-2,4(1H,3H)-dione (9bj), displayed excellent potency against AtHPPD, with an IC50 value of 84 nM, which is approximately 16-fold more potent than pyrasulfotole (IC50 = 1359 nM) and 2.7-fold more potent than mesotrione (IC50 = 226 nM). Furthermore, the co-crystal structure of the AtHPPD-9bj complex (PDB ID 6LGT) was determined at a resolution of 1.75 Å. Similar to the existing HPPD inhibitors, compound 9bj formed a bidentate chelating interaction with the metal ion and a π-π stacking interaction with Phe381 and Phe424. In contrast, o-chlorophenyl at the N3 position of quinazoline-2,4-dione with a double conformation was surrounded by hydrophobic residues (Met335, Leu368, Leu427, Phe424, Phe392, and Phe381). Remarkably, the greenhouse assay indicated that most compounds displayed excellent herbicidal activity (complete inhibition) against at least one of the tested weeds at the application rate of 150 g of active ingredient (ai)/ha. Most promisingly, compounds 9aj and 9bi not only exhibited prominent weed control effects with a broad spectrum but also showed very good crop safety to cotton, peanuts, and corn at the dose of 150 g of ai/ha.
中文翻译:

发现新型吡唑-喹唑啉-2,4-二酮杂化物作为4-羟基苯基丙酮酸双加氧酶抑制剂。
4-羟基苯基丙酮酸双加氧酶(HPPD,EC 1.13.11.27)已被确定为抗除草剂发现中除草剂中最重要的目标之一。为了不断发现有效的新型HPPD抑制剂,我们采用了扩环策略,以先前发现的吡唑-异吲哚啉-1,3-二酮骨架为基础,设计了一系列新型的吡唑-喹唑啉-2,4-二酮杂化物。一种化合物3-(2-氯苯基)-6-(5-羟基-1,3-二甲基-1H-吡唑-4-羰基)-1,5-二甲基喹唑啉-2,4(1H,3H)-二酮( 9bj)显示出对AtHPPD的出色效价,IC50值为84 nM,比吡草磺酚(IC50 = 1359 nM)高约16倍,而对甲基磺草酮(IC50 = 226 nM)则高2.7倍。此外,AtHPPD-9bj复合物(PDB ID 6LGT)的共晶体结构的分辨度为1.75。与现有的HPPD抑制剂相似,化合物9bj与金属离子形成双齿螯合相互作用,与Phe381和Phe424形成π-π堆积相互作用。相反,具有双重构象的喹唑啉-2,4-二酮的N3位置的邻氯苯基被疏水残基(Met335,Leu368,Leu427,Phe424,Phe392和Phe381)包围。值得注意的是,温室试验表明,大多数化合物在150 g活性成分(ai)/ ha的施用量下对至少一种被测杂草表现出优异的除草活性(完全抑制)。最有希望的是,化合物9aj和9bi不仅具有广泛的除草效果,而且对棉花的农作物安全性非常好,
更新日期:2020-04-14
中文翻译:

发现新型吡唑-喹唑啉-2,4-二酮杂化物作为4-羟基苯基丙酮酸双加氧酶抑制剂。
4-羟基苯基丙酮酸双加氧酶(HPPD,EC 1.13.11.27)已被确定为抗除草剂发现中除草剂中最重要的目标之一。为了不断发现有效的新型HPPD抑制剂,我们采用了扩环策略,以先前发现的吡唑-异吲哚啉-1,3-二酮骨架为基础,设计了一系列新型的吡唑-喹唑啉-2,4-二酮杂化物。一种化合物3-(2-氯苯基)-6-(5-羟基-1,3-二甲基-1H-吡唑-4-羰基)-1,5-二甲基喹唑啉-2,4(1H,3H)-二酮( 9bj)显示出对AtHPPD的出色效价,IC50值为84 nM,比吡草磺酚(IC50 = 1359 nM)高约16倍,而对甲基磺草酮(IC50 = 226 nM)则高2.7倍。此外,AtHPPD-9bj复合物(PDB ID 6LGT)的共晶体结构的分辨度为1.75。与现有的HPPD抑制剂相似,化合物9bj与金属离子形成双齿螯合相互作用,与Phe381和Phe424形成π-π堆积相互作用。相反,具有双重构象的喹唑啉-2,4-二酮的N3位置的邻氯苯基被疏水残基(Met335,Leu368,Leu427,Phe424,Phe392和Phe381)包围。值得注意的是,温室试验表明,大多数化合物在150 g活性成分(ai)/ ha的施用量下对至少一种被测杂草表现出优异的除草活性(完全抑制)。最有希望的是,化合物9aj和9bi不仅具有广泛的除草效果,而且对棉花的农作物安全性非常好,






























 京公网安备 11010802027423号
京公网安备 11010802027423号