当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Light Induced Ligation of o-Quinodimethanes with Gated Fluorescence Self-Reporting
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-04-15 , DOI: 10.1021/jacs.0c02002
Florian Feist 1 , Leona L Rodrigues , Sarah L Walden , Tim W Krappitz , Tim R Dargaville , Tanja Weil 1 , Anja S Goldmann , James P Blinco , Christopher Barner-Kowollik
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-04-15 , DOI: 10.1021/jacs.0c02002
Florian Feist 1 , Leona L Rodrigues , Sarah L Walden , Tim W Krappitz , Tim R Dargaville , Tanja Weil 1 , Anja S Goldmann , James P Blinco , Christopher Barner-Kowollik
Affiliation
|
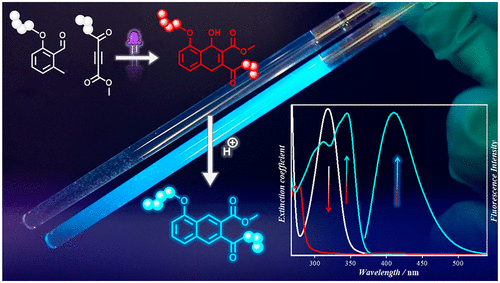
We introduce a highly efficient photoligation system, affording a pro-fluorescent Diels-Alder product which on demand converts into an intensively fluorescent naphthalene via E1-elimination in the presence of catalytic amounts of acid. The Diels-Alder reaction of the photocaged diene (o-quinodimethane ether or thioether, o-QDMs) with electron deficient alkynes is induced by UV or visible light. In contrast to previously reported ligation techniques directly leading to fluorescent products, the fluorescence is turned on after the photoligation. Thus, the light absorption of the fluorophore does not undermine the photoligation via competitive absorption and as a result, photobleaching or side reactions of the fluorophore are not observed. Critically, the gated generation of a fluorescent product allows for fluorometric determination of the conversion. We employ a simple synthesis strategy for heterobifunctional electron deficient alkynes allowing for facile functionalization of payload molecules.
中文翻译:

o-Quinodimethanes 的光诱导连接与门控荧光自报告
我们引入了一种高效的光连接系统,提供了一种亲荧光的 Diels-Alder 产品,该产品可根据需要在催化量的酸存在下通过 E1 消除转化为强荧光的萘。光笼化二烯(邻醌二甲烷醚或硫醚,o-QDM)与缺电子炔烃的 Diels-Alder 反应是由紫外线或可见光引起的。与先前报道的直接导致荧光产物的结扎技术相比,荧光在光结扎后打开。因此,荧光团的光吸收不会通过竞争性吸收破坏光连接,因此不会观察到荧光团的光漂白或副反应。关键的是,荧光产物的门控生成允许对转化进行荧光测定。我们对异双功能缺电子炔烃采用了一种简单的合成策略,可以轻松实现有效载荷分子的功能化。
更新日期:2020-04-15
中文翻译:

o-Quinodimethanes 的光诱导连接与门控荧光自报告
我们引入了一种高效的光连接系统,提供了一种亲荧光的 Diels-Alder 产品,该产品可根据需要在催化量的酸存在下通过 E1 消除转化为强荧光的萘。光笼化二烯(邻醌二甲烷醚或硫醚,o-QDM)与缺电子炔烃的 Diels-Alder 反应是由紫外线或可见光引起的。与先前报道的直接导致荧光产物的结扎技术相比,荧光在光结扎后打开。因此,荧光团的光吸收不会通过竞争性吸收破坏光连接,因此不会观察到荧光团的光漂白或副反应。关键的是,荧光产物的门控生成允许对转化进行荧光测定。我们对异双功能缺电子炔烃采用了一种简单的合成策略,可以轻松实现有效载荷分子的功能化。































 京公网安备 11010802027423号
京公网安备 11010802027423号