当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Hydrogenation and Oxidation in Aqueous Conditions†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-04-14 , DOI: 10.1002/cjoc.201900467
Peili Zhang 1 , Licheng Sun 1, 2
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-04-14 , DOI: 10.1002/cjoc.201900467
Peili Zhang 1 , Licheng Sun 1, 2
Affiliation
|
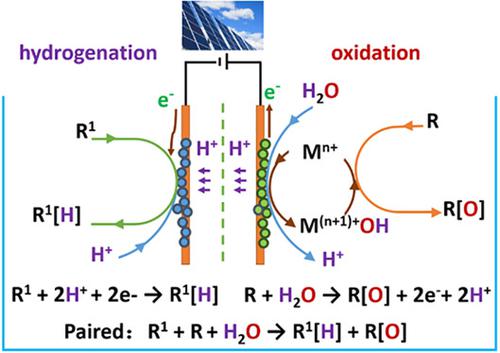
Water molecule contains one oxygen and two hydrogen atoms, making it a potential oxygen and hydrogen source. Electrocatalytic organic reduction and oxidation using water as oxygen and/or hydrogen donors provide an environmentally friendly and sustainable strategy to replace traditional chemical‐driven stoichiometric reactions that use sacrificial reagents. Furthermore, the development of electrochemical synthesis provides a potential application for low tension photoelectricity, which is not cost‐effective during boosted voltage and application. In the last decade, electrocatalytic redox reactions of organic molecules in aqueous media had shown progress owing to the development of electrode materials and water‐splitting technology. This paper highlights several electrocatalytic systems and corresponding mechanisms for both hydrogenation and oxidative transformation of representative compounds. The activation process of protons and water on the working electrode surface has received special focus. Furthermore, paired electrolysis using water as the oxygen and hydrogen source has been demonstrated. This paired system combines hydrogenation and oxidation half‐reactions in one cell using water as the hydrogen and oxygen source, resulting in high atomic and electron utilization rates.
中文翻译:

水溶液中的电催化加氢和氧化†
水分子包含一个氧和两个氢原子,使其成为潜在的氧和氢源。使用水作为氧和/或氢供体的电催化有机还原和氧化提供了一种环境友好和可持续的策略,以取代使用牺牲试剂的传统化学驱动化学计量反应。此外,电化学合成技术的发展为低张力光电技术提供了潜在的应用,这在提高电压和应用过程中并不划算。在过去的十年中,由于电极材料和水分解技术的发展,有机分子在水介质中的电催化氧化还原反应取得了进展。本文着重介绍了几种代表性化合物的电催化体系及其加氢和氧化转化的相应机理。质子和水在工作电极表面的活化过程受到了特别关注。此外,已经证明了使用水作为氧和氢源的配对电解。这种配对的系统将水和氢作为氧和氧的来源,在一个单元中结合了氢化和氧化半反应,从而提高了原子和电子的利用率。
更新日期:2020-04-14

中文翻译:

水溶液中的电催化加氢和氧化†
水分子包含一个氧和两个氢原子,使其成为潜在的氧和氢源。使用水作为氧和/或氢供体的电催化有机还原和氧化提供了一种环境友好和可持续的策略,以取代使用牺牲试剂的传统化学驱动化学计量反应。此外,电化学合成技术的发展为低张力光电技术提供了潜在的应用,这在提高电压和应用过程中并不划算。在过去的十年中,由于电极材料和水分解技术的发展,有机分子在水介质中的电催化氧化还原反应取得了进展。本文着重介绍了几种代表性化合物的电催化体系及其加氢和氧化转化的相应机理。质子和水在工作电极表面的活化过程受到了特别关注。此外,已经证明了使用水作为氧和氢源的配对电解。这种配对的系统将水和氢作为氧和氧的来源,在一个单元中结合了氢化和氧化半反应,从而提高了原子和电子的利用率。


































 京公网安备 11010802027423号
京公网安备 11010802027423号