当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chromatin architecture reorganization in murine somatic cell nuclear transfer embryos.
Nature Communications ( IF 14.7 ) Pub Date : 2020-04-14 , DOI: 10.1038/s41467-020-15607-z Mo Chen 1, 2, 3, 4, 5 , Qianshu Zhu 2 , Chong Li 1 , Xiaochen Kou 1 , Yanhong Zhao 1 , Yanhe Li 1 , Ruimin Xu 1 , Lei Yang 1 , Lingyue Yang 1 , Liang Gu 2 , Hong Wang 1 , Xiaoyu Liu 3 , Cizhong Jiang 2 , Shaorong Gao 1, 3
Nature Communications ( IF 14.7 ) Pub Date : 2020-04-14 , DOI: 10.1038/s41467-020-15607-z Mo Chen 1, 2, 3, 4, 5 , Qianshu Zhu 2 , Chong Li 1 , Xiaochen Kou 1 , Yanhong Zhao 1 , Yanhe Li 1 , Ruimin Xu 1 , Lei Yang 1 , Lingyue Yang 1 , Liang Gu 2 , Hong Wang 1 , Xiaoyu Liu 3 , Cizhong Jiang 2 , Shaorong Gao 1, 3
Affiliation
|
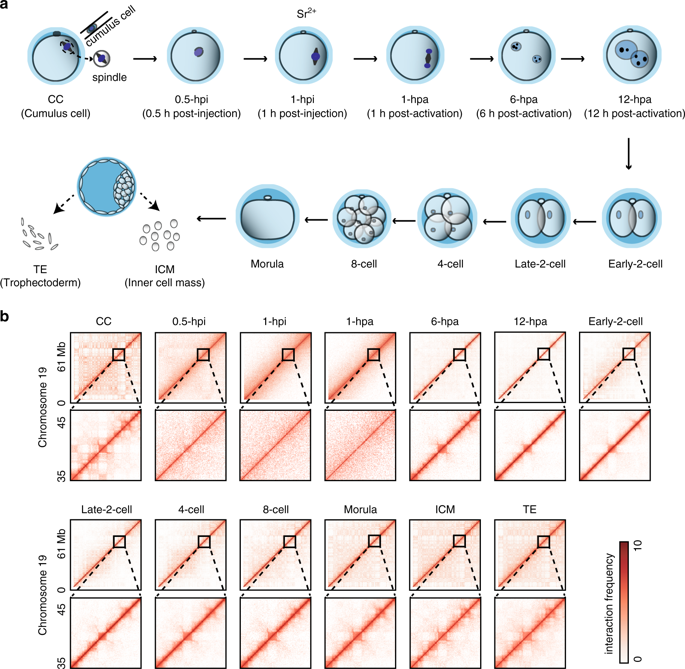
The oocyte cytoplasm can reprogram the somatic cell nucleus into a totipotent state, but with low efficiency. The spatiotemporal chromatin organization of somatic cell nuclear transfer (SCNT) embryos remains elusive. Here, we examine higher order chromatin structures of mouse SCNT embryos using a low-input Hi-C method. We find that donor cell chromatin transforms to the metaphase state rapidly after SCNT along with the dissolution of typical 3D chromatin structure. Intriguingly, the genome undergoes a mitotic metaphase-like to meiosis metaphase II-like transition following activation. Subsequently, weak chromatin compartments and topologically associating domains (TADs) emerge following metaphase exit. TADs are further removed until the 2-cell stage before being progressively reestablished. Obvious defects including stronger TAD boundaries, aberrant super-enhancer and promoter interactions are found in SCNT embryos. These defects are partially caused by inherited H3K9me3, and can be rescued by Kdm4d overexpression. These observations provide insight into chromatin architecture reorganization during SCNT embryo development.
中文翻译:

鼠体细胞核移植胚胎中的染色质结构重组。
卵母细胞的细胞质可以将体细胞核重编程为全能状态,但效率较低。体细胞核移植(SCNT)胚胎的时空染色质组织仍然难以捉摸。在这里,我们检查使用低输入Hi-C方法的小鼠SCNT胚胎的高阶染色质结构。我们发现供体细胞染色质随着典型3D染色质结构的溶解而迅速转变为SCNT后的中期状态。有趣的是,基因组在激活后经历了从有丝分裂中期到减数分裂中期II的过渡。随后,弱染色质区室和拓扑关联域(TADs)在中期退出后出现。在逐步重建之前,将TAD进一步移除直到2单元阶段。明显的缺陷包括更强的TAD边界,SCNT胚胎中发现异常的超级增强子和启动子相互作用。这些缺陷部分是由继承的H3K9me3引起的,并且可以通过Kdm4d过表达来挽救。这些观察结果提供了SCNT胚胎发育过程中染色质结构重组的见解。
更新日期:2020-04-24
中文翻译:

鼠体细胞核移植胚胎中的染色质结构重组。
卵母细胞的细胞质可以将体细胞核重编程为全能状态,但效率较低。体细胞核移植(SCNT)胚胎的时空染色质组织仍然难以捉摸。在这里,我们检查使用低输入Hi-C方法的小鼠SCNT胚胎的高阶染色质结构。我们发现供体细胞染色质随着典型3D染色质结构的溶解而迅速转变为SCNT后的中期状态。有趣的是,基因组在激活后经历了从有丝分裂中期到减数分裂中期II的过渡。随后,弱染色质区室和拓扑关联域(TADs)在中期退出后出现。在逐步重建之前,将TAD进一步移除直到2单元阶段。明显的缺陷包括更强的TAD边界,SCNT胚胎中发现异常的超级增强子和启动子相互作用。这些缺陷部分是由继承的H3K9me3引起的,并且可以通过Kdm4d过表达来挽救。这些观察结果提供了SCNT胚胎发育过程中染色质结构重组的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号