Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cell surface processing of the P1 adhesin of Mycoplasma pneumoniae identifies novel domains that bind host molecules.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-04-14 , DOI: 10.1038/s41598-020-63136-y Michael Widjaja 1 , Iain James Berry 1 , Veronica Maria Jarocki 1 , Matthew Paul Padula 2 , Roger Dumke 3 , Steven Philip Djordjevic 1, 2
Scientific Reports ( IF 3.8 ) Pub Date : 2020-04-14 , DOI: 10.1038/s41598-020-63136-y Michael Widjaja 1 , Iain James Berry 1 , Veronica Maria Jarocki 1 , Matthew Paul Padula 2 , Roger Dumke 3 , Steven Philip Djordjevic 1, 2
Affiliation
|
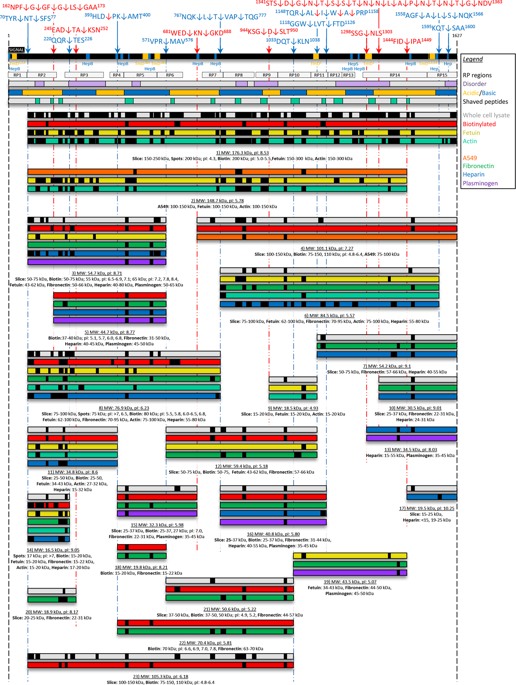
Mycoplasma pneumoniae is a genome reduced pathogen and causative agent of community acquired pneumonia. The major cellular adhesin, P1, localises to the tip of the attachment organelle forming a complex with P40 and P90, two cleavage fragments derived by processing Mpn142, and other molecules with adhesive and mobility functions. LC-MS/MS analysis of M. pneumoniae M129 proteins derived from whole cell lysates and eluents from affinity matrices coupled with chemically diverse host molecules identified 22 proteoforms of P1. Terminomics was used to characterise 17 cleavage events many of which were independently verified by the identification of semi-tryptic peptides in our proteome studies and by immunoblotting. One cleavage event released 1597TSAAKPGAPRPPVPPKPGAPKPPVQPPKKPA1627 from the C-terminus of P1 and this peptide was shown to bind to a range of host molecules. A smaller synthetic peptide comprising the C-terminal 15 amino acids, 1613PGAPKPPVQPPKKPA1627, selectively bound cytoskeletal intermediate filament proteins cytokeratin 7, cytokeratin 8, cytokeratin 18, and vimentin from a native A549 cell lysate. Collectively, our data suggests that ectodomain shedding occurs on the surface of M. pneumoniae where it may alter the functional diversity of P1, Mpn142 and other surface proteins such as elongation factor Tu via a mechanism similar to that described in Mycoplasma hyopneumoniae.
中文翻译:

肺炎支原体的P1粘附素的细胞表面加工鉴定出结合宿主分子的新结构域。
肺炎支原体是基因组减少的病原体和社区获得性肺炎的病原体。主要的细胞粘附素P1位于附着细胞器的末端,与P40和P90,通过加工Mpn142衍生的两个切割片段以及具有粘附和迁移功能的其他分子形成复合物。对全细胞裂解物和亲和基质中的洗脱液以及化学上不同的宿主分子结合的肺炎支原体M129蛋白进行LC-MS / MS分析,鉴定出22种P1蛋白。术语组学用于表征17个裂解事件,其中许多事件在我们的蛋白质组研究中通过鉴定半胰蛋白酶肽和通过免疫印迹而得到独立验证。一个裂解事件从P1的C末端释放了1597TSAAKPGAPRPPVPPKPGAPKPPVQPPKKPA1627,并且该肽显示与一系列宿主分子结合。较小的合成肽,包含C端15个氨基酸1613PGAPKPPVQPPKKPA1627,可选择性结合天然A549细胞裂解物中的细胞骨架中间细丝蛋白细胞角蛋白7,细胞角蛋白8,细胞角蛋白18和波形蛋白。总的来说,我们的数据表明,胞外域脱落发生在肺炎支原体的表面,通过类似于肺炎支原体中所述的机制,它可能会改变P1,Mpn142和其他表面蛋白(如延伸因子Tu)的功能多样性。从天然A549细胞裂解物中选择性结合细胞骨架中间细丝蛋白细胞角蛋白7,细胞角蛋白8,细胞角蛋白18和波形蛋白。总体而言,我们的数据表明,胞外域脱落发生在肺炎支原体的表面,在那里它可能通过类似于猪肺炎支原体中所述的机制改变P1,Mpn142和其他表面蛋白(例如延伸因子Tu)的功能多样性。从天然A549细胞裂解物中选择性结合细胞骨架中间细丝蛋白细胞角蛋白7,细胞角蛋白8,细胞角蛋白18和波形蛋白。总体而言,我们的数据表明,胞外域脱落发生在肺炎支原体的表面,在那里它可能通过类似于猪肺炎支原体中所述的机制改变P1,Mpn142和其他表面蛋白(例如延伸因子Tu)的功能多样性。
更新日期:2020-04-14
中文翻译:

肺炎支原体的P1粘附素的细胞表面加工鉴定出结合宿主分子的新结构域。
肺炎支原体是基因组减少的病原体和社区获得性肺炎的病原体。主要的细胞粘附素P1位于附着细胞器的末端,与P40和P90,通过加工Mpn142衍生的两个切割片段以及具有粘附和迁移功能的其他分子形成复合物。对全细胞裂解物和亲和基质中的洗脱液以及化学上不同的宿主分子结合的肺炎支原体M129蛋白进行LC-MS / MS分析,鉴定出22种P1蛋白。术语组学用于表征17个裂解事件,其中许多事件在我们的蛋白质组研究中通过鉴定半胰蛋白酶肽和通过免疫印迹而得到独立验证。一个裂解事件从P1的C末端释放了1597TSAAKPGAPRPPVPPKPGAPKPPVQPPKKPA1627,并且该肽显示与一系列宿主分子结合。较小的合成肽,包含C端15个氨基酸1613PGAPKPPVQPPKKPA1627,可选择性结合天然A549细胞裂解物中的细胞骨架中间细丝蛋白细胞角蛋白7,细胞角蛋白8,细胞角蛋白18和波形蛋白。总的来说,我们的数据表明,胞外域脱落发生在肺炎支原体的表面,通过类似于肺炎支原体中所述的机制,它可能会改变P1,Mpn142和其他表面蛋白(如延伸因子Tu)的功能多样性。从天然A549细胞裂解物中选择性结合细胞骨架中间细丝蛋白细胞角蛋白7,细胞角蛋白8,细胞角蛋白18和波形蛋白。总体而言,我们的数据表明,胞外域脱落发生在肺炎支原体的表面,在那里它可能通过类似于猪肺炎支原体中所述的机制改变P1,Mpn142和其他表面蛋白(例如延伸因子Tu)的功能多样性。从天然A549细胞裂解物中选择性结合细胞骨架中间细丝蛋白细胞角蛋白7,细胞角蛋白8,细胞角蛋白18和波形蛋白。总体而言,我们的数据表明,胞外域脱落发生在肺炎支原体的表面,在那里它可能通过类似于猪肺炎支原体中所述的机制改变P1,Mpn142和其他表面蛋白(例如延伸因子Tu)的功能多样性。































 京公网安备 11010802027423号
京公网安备 11010802027423号