Molecular Therapy ( IF 12.1 ) Pub Date : 2020-04-14 , DOI: 10.1016/j.ymthe.2020.04.006
Annadoray Lavenniah 1 , Tuan Danh Anh Luu 2 , Yiqing Peter Li 2 , Tingsen Benson Lim 1 , Jianming Jiang 2 , Matthew Ackers-Johnson 1 , Roger S-Y Foo 1
|
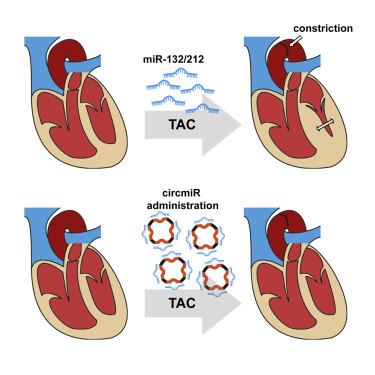
Circular RNAs (circRNAs) sequester microRNAs (miRNAs) and repress their endogenous activity. We hypothesized that artificial circRNA sponges (circmiRs) can be constructed to target miRNAs therapeutically, with a low dosage requirement and extended half-lives compared to current alternatives. This could present a new treatment approach for critical global pathologies, including cardiovascular disease. Here, we constructed a circmiR sponge to target known cardiac pro-hypertrophic miR-132 and -212. Expressed circmiRs competitively inhibited miR-132 and -212 activity in luciferase rescue assays and showed greater stability than linear sponges. A design containing 12 bulged binding sites with 12 nucleotides spacing was determined to be optimal. Adeno-associated viruses (AAVs) were used to deliver circmiRs to cardiomyocytes in vivo in a transverse aortic constriction (TAC) mouse model of cardiac disease. Hypertrophic disease characteristics were attenuated, and cardiac function was preserved in treated mice, demonstrating the potential of circmiRs as novel therapeutic tools. Subsequently, group I permutated intron-exon sequences were used to directly synthesize exogenous circmiRs, which showed greater in vitro efficacy than the current gold standard antagomiRs in inhibiting miRNA function. Engineered circRNAs thus offer exciting potential as future therapeutics.
中文翻译:

工程环形RNA海绵可作为miRNA抑制剂来减轻压力超负荷引起的心肌肥大。
环状RNA(circRNA)隔离microRNA(miRNA),并抑制其内源活性。我们假设人造circRNA海绵(circmiRs)可以构建为治疗性靶向miRNA,与目前的替代品相比,剂量要求低且半衰期延长。这可以为包括心血管疾病在内的关键性全球疾病提供一种新的治疗方法。在这里,我们构建了circmiR海绵,以靶向已知的心脏肥大前体miR-132和-212。在荧光素酶拯救试验中,表达的circmiRs竞争性抑制miR-132和-212活性,并且比线性海绵具有更高的稳定性。确定包含12个凸起的结合位点且间距为12个核苷酸的设计是最佳的。腺相关病毒(AAV)用于将circmiR传递至心肌细胞在心脏疾病的横向主动脉缩窄(TAC)小鼠模型中进行体内研究。肥厚性疾病的特征减弱,并且在治疗的小鼠中心脏功能得以保留,证明了circmiRs作为新型治疗工具的潜力。随后,使用第I组置换的内含子-外显子序列直接合成外源性circmiRs,其在抑制miRNA功能方面比目前的金标准antagomiRs具有更高的体外功效。因此,工程circRNA具有令人兴奋的潜力,可作为未来的治疗方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号