Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-04-14 , DOI: 10.1016/j.jechem.2020.03.083 Xinyu Jia , Kaihang Sun , Jing Wang , Chenyang Shen , Chang-jun Liu
|
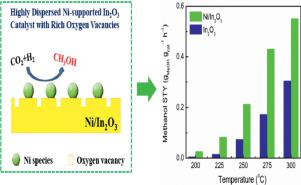
An In2O3 supported nickel catalyst has been prepared by wet chemical reduction with sodium borohydride (NaBH4) as a reducing agent for selective hydrogenation of carbon dioxide to methanol. Highly dispersed Ni species with intense Ni-In2O3 interaction and enhanced oxygen vacancies have been achieved. The highly dispersed Ni species serve as the active sites for hydrogen activation and hydrogen spillover. Abundant H adatoms are thereby generated for the oxygen vacancy creation on the In2O3 surface. The enhanced surface oxygen vacancies further lead to improved CO2 conversion. As a result, an effective synergy between the active Ni sites and surface oxygen vacancies on In2O3 causes a superior catalytic performance for CO2 hydrogenation with high methanol selectivity. Carbon monoxide is the only by product detected. The formation of methane can be ignored. When the reaction temperature is lower than 225 °C, the selectivity of methanol is 100%. It is higher than 64% at the temperature range between 225 °C and 275 °C. The methanol selectivity is still higher than 54% at 300 °C with a CO2 conversion of 18.47% and a methanol yield of 0.55 gMeOH gcat−1 h−1 (at 5 MPa). The activity of Ni/In2O3 is higher than most of the reported In2O3-based catalysts.
中文翻译:

在Ni / In 2 O 3催化剂上将CO 2选择性加氢为甲醇
通过用硼氢化钠(NaBH 4)作为用于二氧化碳选择性加氢成甲醇的还原剂进行湿式化学还原,制备了由In 2 O 3负载的镍催化剂。已实现具有高度的Ni-In 2 O 3相互作用和增强的氧空位的高度分散的Ni物种。高度分散的镍物质充当氢活化和氢溢出的活性位点。由此产生大量的H原子,以在In 2 O 3表面上产生氧空位。表面氧空位的增加进一步导致CO 2的改善转换。结果,活性Ni位点和In 2 O 3上的表面氧空位之间的有效协同作用导致具有高甲醇选择性的CO 2加氢的优异催化性能。一氧化碳是唯一检测到的副产物。甲烷的形成可以忽略。当反应温度低于225℃时,甲醇的选择性为100%。在225°C至275°C的温度范围内,它高于64%。在300°C下,甲醇的选择性仍高于54%,CO 2转化率为18.47%,甲醇产率为0.55 g MeOH g cat -1 h -1(在5 MPa下)。Ni / In 2的活性O 3高于大多数报道的基于In 2 O 3的催化剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号