当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charging Mechanism of Li2MnO3
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-04-14 , DOI: 10.1021/acs.chemmater.9b04459
Niccoló Guerrini 1 , Liyu Jin 1 , Juan G. Lozano 2 , Kun Luo 2 , Adam Sobkowiak 3 , Kazuki Tsuruta 4 , Felix Massel 5 , Laurent-Claudius Duda 5 , Matthew R. Roberts 1 , Peter G. Bruce 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-04-14 , DOI: 10.1021/acs.chemmater.9b04459
Niccoló Guerrini 1 , Liyu Jin 1 , Juan G. Lozano 2 , Kun Luo 2 , Adam Sobkowiak 3 , Kazuki Tsuruta 4 , Felix Massel 5 , Laurent-Claudius Duda 5 , Matthew R. Roberts 1 , Peter G. Bruce 2
Affiliation
|
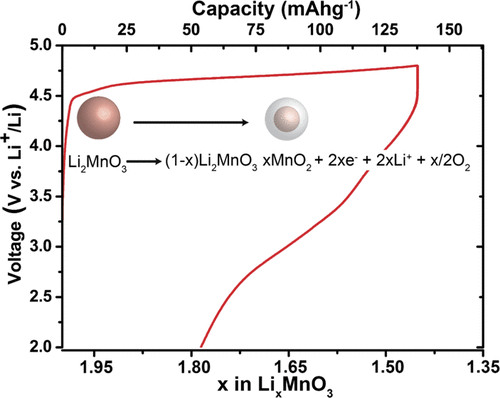
Operando mass spectroscopy demonstrates quantitatively that lithium extraction from Li2MnO3 is charge compensated by oxygen loss (O-loss) not oxidation of oxide ions that are retained within the structural framework (O-redox). This fact is confirmed by X-ray absorption and emission spectroscopy. Li NMR shows that the two-phase core–shell structure, which forms on charging, is composed of an intact Li2MnO3 core and a highly disordered shell containing no Li, with a composition close to MnO2. Discharge involves Li insertion into the disordered shell. CO2 and O2 are detected on charging at 15 mA g–1, whereas charging by galvanostatic intermittent titration technique (GITT) forms only CO2; an observation in agreement with the previously described model of oxygen evolution from high-voltage cathodes producing singlet O2 that reacts with the electrolyte forming CO2. The dominance of oxygen evolution over O-redox is in accordance with the model of O-loss occurring when the oxide ions are undercoordinated; O in the shell devoid of Li is coordinated by only 2 Mn.
中文翻译:

Li 2 MnO 3的充电机理
Operando质谱定量地表明,从Li 2 MnO 3中提取锂是通过氧损失(O-损失)而不是保留在结构框架中的氧离子的氧化(O-氧化还原)来补偿电荷的。X射线吸收和发射光谱证实了这一事实。Li NMR显示,在充电时形成的两相核-壳结构由完整的Li 2 MnO 3核和不含锂的高度无序的壳组成,其组成接近MnO 2。放电涉及将锂插入无序壳中。以15 mA g –1充电时检测到CO 2和O 2,而通过恒电流间歇滴定技术(GITT)充电仅形成CO 2;观察结果与先前描述的从高压阴极放出氧气产生单线态O 2的模型相符,单线态O 2与形成CO 2的电解质反应。氧在O-氧化还原上的支配性是根据当氧化物离子配位不足时发生的O-损失模型确定的。不含Li的壳中的O仅由2 Mn配位。
更新日期:2020-04-14
中文翻译:

Li 2 MnO 3的充电机理
Operando质谱定量地表明,从Li 2 MnO 3中提取锂是通过氧损失(O-损失)而不是保留在结构框架中的氧离子的氧化(O-氧化还原)来补偿电荷的。X射线吸收和发射光谱证实了这一事实。Li NMR显示,在充电时形成的两相核-壳结构由完整的Li 2 MnO 3核和不含锂的高度无序的壳组成,其组成接近MnO 2。放电涉及将锂插入无序壳中。以15 mA g –1充电时检测到CO 2和O 2,而通过恒电流间歇滴定技术(GITT)充电仅形成CO 2;观察结果与先前描述的从高压阴极放出氧气产生单线态O 2的模型相符,单线态O 2与形成CO 2的电解质反应。氧在O-氧化还原上的支配性是根据当氧化物离子配位不足时发生的O-损失模型确定的。不含Li的壳中的O仅由2 Mn配位。







































 京公网安备 11010802027423号
京公网安备 11010802027423号