当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Reduction of CO2 to Acetic Acid by a Molecular Manganese Corrole Complex.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-04-13 , DOI: 10.1002/anie.202000601 Ratnadip De 1 , Sabrina Gonglach 2 , Shounik Paul 1 , Michael Haas 2 , S S Sreejith 1 , Philipp Gerschel 3 , Ulf-Peter Apfel 3, 4 , Thanh Huyen Vuong 5 , Jabor Rabeah 5 , Soumyajit Roy 1 , Wolfgang Schöfberger 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-04-13 , DOI: 10.1002/anie.202000601 Ratnadip De 1 , Sabrina Gonglach 2 , Shounik Paul 1 , Michael Haas 2 , S S Sreejith 1 , Philipp Gerschel 3 , Ulf-Peter Apfel 3, 4 , Thanh Huyen Vuong 5 , Jabor Rabeah 5 , Soumyajit Roy 1 , Wolfgang Schöfberger 2
Affiliation
|
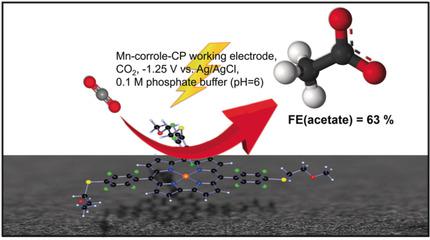
The controlled electrochemical reduction of carbon dioxide to value added chemicals is an important strategy in terms of renewable energy technologies. Therefore, the development of efficient and stable catalysts in an aqueous environment is of great importance. In this context, we focused on synthesizing and studying a molecular MnIII‐corrole complex, which is modified on the three meso ‐positions with polyethylene glycol moieties for direct and selective production of acetic acid from CO2. Electrochemical reduction of MnIII leads to an electroactive MnII species, which binds CO2 and stabilizes the reduced intermediates. This catalyst allows to electrochemically reduce CO2 to acetic acid in a moderate acidic aqueous medium (pH 6) with a selectivity of 63 % and a turn over frequency (TOF) of 8.25 h−1, when immobilized on a carbon paper (CP) electrode. In terms of high selectivity towards acetate, we propose the formation and reduction of an oxalate type intermediate, stabilized at the MnIII‐corrole center.
中文翻译:

分子锰配合物将CO2电催化还原为乙酸。
就可再生能源技术而言,受控地将二氧化碳电化学还原为增值化学品是一项重要策略。因此,在水性环境中开发有效和稳定的催化剂非常重要。在这种情况下,我们专注于合成和研究分子Mn III分子复合物,该复合物在三个内消旋位上被聚乙二醇部分修饰,可以直接和选择性地从CO 2中生产乙酸。Mn III的电化学还原产生电活性Mn II物质,该物质结合CO 2并稳定还原的中间体。该催化剂可以电化学还原CO当固定在碳纸(CP)电极上时,在中等酸性水性介质(pH 6)中具有2的乙酸选择性,选择性为63%,转换频率(TOF)为8.25 h -1。就对乙酸盐的高选择性而言,我们建议形成并还原一种稳定在Mn III- Corrole中心的草酸盐型中间体。
更新日期:2020-04-13
中文翻译:

分子锰配合物将CO2电催化还原为乙酸。
就可再生能源技术而言,受控地将二氧化碳电化学还原为增值化学品是一项重要策略。因此,在水性环境中开发有效和稳定的催化剂非常重要。在这种情况下,我们专注于合成和研究分子Mn III分子复合物,该复合物在三个内消旋位上被聚乙二醇部分修饰,可以直接和选择性地从CO 2中生产乙酸。Mn III的电化学还原产生电活性Mn II物质,该物质结合CO 2并稳定还原的中间体。该催化剂可以电化学还原CO当固定在碳纸(CP)电极上时,在中等酸性水性介质(pH 6)中具有2的乙酸选择性,选择性为63%,转换频率(TOF)为8.25 h -1。就对乙酸盐的高选择性而言,我们建议形成并还原一种稳定在Mn III- Corrole中心的草酸盐型中间体。













































 京公网安备 11010802027423号
京公网安备 11010802027423号