当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of a human prion fibril with a hydrophobic, protease-resistant core.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2020-04-13 , DOI: 10.1038/s41594-020-0403-y Calina Glynn 1 , Michael R Sawaya 2 , Peng Ge 3 , Marcus Gallagher-Jones 1 , Connor W Short 1 , Ronquiajah Bowman 1 , Marcin Apostol 2, 4 , Z Hong Zhou 3, 5 , David S Eisenberg 2 , Jose A Rodriguez 1
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2020-04-13 , DOI: 10.1038/s41594-020-0403-y Calina Glynn 1 , Michael R Sawaya 2 , Peng Ge 3 , Marcus Gallagher-Jones 1 , Connor W Short 1 , Ronquiajah Bowman 1 , Marcin Apostol 2, 4 , Z Hong Zhou 3, 5 , David S Eisenberg 2 , Jose A Rodriguez 1
Affiliation
|
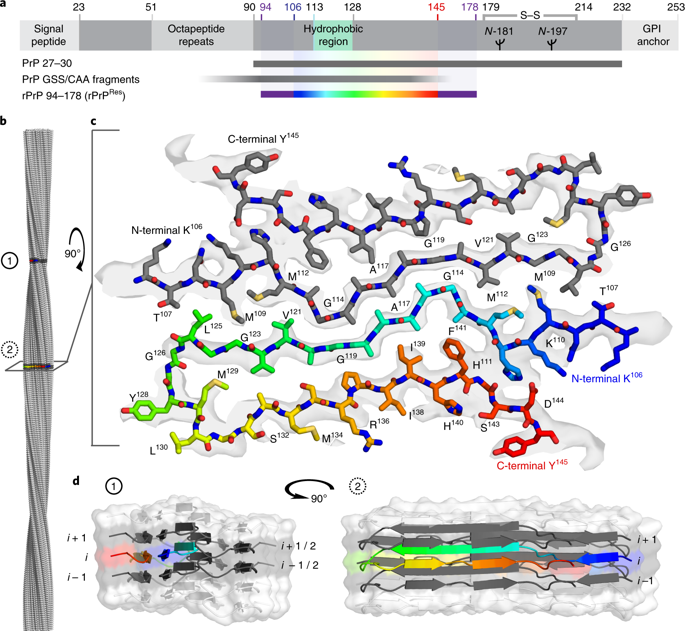
Self-templating assemblies of the human prion protein are clinically associated with transmissible spongiform encephalopathies. Here we present the cryo-EM structure of a denaturant- and protease-resistant fibril formed in vitro spontaneously by a 9.7-kDa unglycosylated fragment of the human prion protein. This human prion fibril contains two protofilaments intertwined with screw symmetry and linked by a tightly packed hydrophobic interface. Each protofilament consists of an extended beta arch formed by residues 106 to 145 of the prion protein, a hydrophobic and highly fibrillogenic disease-associated segment. Such structures of prion polymorphs serve as blueprints on which to evaluate the potential impact of sequence variants on prion disease.
中文翻译:

具有疏水、耐蛋白酶核心的人朊病毒原纤维的冷冻电镜结构。
人类朊病毒蛋白的自模板组装在临床上与传染性海绵状脑病相关。在这里,我们展示了由人朊病毒蛋白的 9.7 kDa 未糖基化片段在体外自发形成的抗变性剂和蛋白酶原纤维的冷冻电镜结构。这种人类朊病毒原纤维包含两条以螺旋对称方式交织在一起的原丝,并通过紧密堆积的疏水界面连接。每个原丝由由朊病毒蛋白的残基 106 至 145 形成的延伸 β 弓组成,朊病毒蛋白是疏水性且高度纤维化的疾病相关片段。朊病毒多态性的此类结构可作为评估序列变异对朊病毒疾病的潜在影响的蓝图。
更新日期:2020-04-13
中文翻译:

具有疏水、耐蛋白酶核心的人朊病毒原纤维的冷冻电镜结构。
人类朊病毒蛋白的自模板组装在临床上与传染性海绵状脑病相关。在这里,我们展示了由人朊病毒蛋白的 9.7 kDa 未糖基化片段在体外自发形成的抗变性剂和蛋白酶原纤维的冷冻电镜结构。这种人类朊病毒原纤维包含两条以螺旋对称方式交织在一起的原丝,并通过紧密堆积的疏水界面连接。每个原丝由由朊病毒蛋白的残基 106 至 145 形成的延伸 β 弓组成,朊病毒蛋白是疏水性且高度纤维化的疾病相关片段。朊病毒多态性的此类结构可作为评估序列变异对朊病毒疾病的潜在影响的蓝图。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号