当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Applications of 2-Chloro-3,3,3-trifluoroprop-1-ene (HCFO-1233xf): A Rapid Entry to Various β-Substituted-trifluoromethyl-ethenes.
Organic Letters ( IF 4.9 ) Pub Date : 2020-04-13 , DOI: 10.1021/acs.orglett.0c00931 Daniel Meyer 1 , Myriem El Qacemi 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-04-13 , DOI: 10.1021/acs.orglett.0c00931 Daniel Meyer 1 , Myriem El Qacemi 1
Affiliation
|
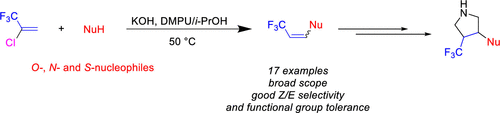
An efficient base-promoted reaction of O-, N-, and S-nucleophiles with 2-chloro-3,3,3-trifluoprop-1-ene (HCFO-1233xf) is described providing access to various β-substituted-trifluoromethyl-ethenes under mild reaction conditions. Mechanistic investigations shed some light on the regio-, chemo-, and stereoselectivities observed. The olefins prepared represent attractive intermediates in chemical discovery: some applications include their conversion to pyrrolidines via a [3 + 2] dipolar cycloaddition reaction. These weakly basic amines represent novel synthons that could be readily elaborated through a range of reactions.
中文翻译:

2-氯-3,3,3-三氟丙-1-烯(HCFO-1233xf)的应用:各种β-取代的三氟甲基乙烯的快速入门。
描述了O-,N-和S-亲核试剂与2-氯-3,3,3-三氟丙-1-烯(HCFO-1233xf)的有效碱促进反应,可提供各种β-取代的三氟甲基-乙烯在温和的反应条件下。机理研究为观察到的区域选择性,化学选择性和立体选择性提供了一些启示。制备的烯烃代表了化学发现中的诱人中间体:某些应用包括通过[3 + 2]偶极环加成反应将其转化为吡咯烷。这些弱碱性胺代表了新型合成子,可以通过一系列反应轻松地进行合成。
更新日期:2020-04-13
中文翻译:

2-氯-3,3,3-三氟丙-1-烯(HCFO-1233xf)的应用:各种β-取代的三氟甲基乙烯的快速入门。
描述了O-,N-和S-亲核试剂与2-氯-3,3,3-三氟丙-1-烯(HCFO-1233xf)的有效碱促进反应,可提供各种β-取代的三氟甲基-乙烯在温和的反应条件下。机理研究为观察到的区域选择性,化学选择性和立体选择性提供了一些启示。制备的烯烃代表了化学发现中的诱人中间体:某些应用包括通过[3 + 2]偶极环加成反应将其转化为吡咯烷。这些弱碱性胺代表了新型合成子,可以通过一系列反应轻松地进行合成。








































 京公网安备 11010802027423号
京公网安备 11010802027423号