当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of tumor microenvironment in the regulation of PD-L1: A novel role in resistance to cancer immunotherapy.
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-04-02 , DOI: 10.1002/jcp.29671 Nirvana Kalantari Khandani 1 , Atefeh Ghahremanloo 1, 2 , Seyed Isaac Hashemy 1, 3
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-04-02 , DOI: 10.1002/jcp.29671 Nirvana Kalantari Khandani 1 , Atefeh Ghahremanloo 1, 2 , Seyed Isaac Hashemy 1, 3
Affiliation
|
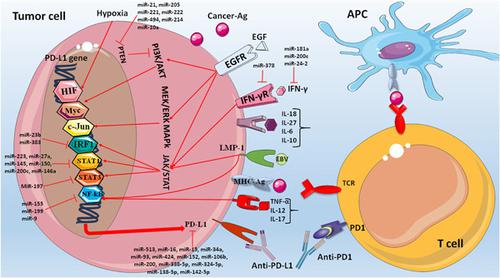
Tumor evasion from the host immune system is a substantial strategy for tumor development and survival. The expression of many immune checkpoint proteins in cancer cells is a mechanism by which tumor cells escape from the immune system. Among the well‐known immune checkpoints that can tremendously affect tumor development and cancer therapy are the programmed death‐ligand‐1/programmed death‐1 (PD‐L1/PD‐1). To tackle this phenomenon and improve the therapeutic strategies in cancer treatment, the blockade of the PD‐L1/PD‐1 pathway is introduced as a target, but the therapeutic advantage of PD L1/PD‐1 blockade has not fulfilled the expectations. This condition may be associated with a different type of resistance in a considerable number of patients. A crucial issue to conquer resistance against immune checkpoint blockade therapy is to understand how PD‐L1 level is regulated. However, the mechanisms by which the PD‐L1 expression is regulated are complicated, and they can occur at different levels from signaling pathways to posttranscriptional levels. For example, various transcriptional factors, such as hypoxia‐inducible factor‐1, nuclear factor‐κΒ, interferon‐γ, STAT3, MYC, and AP‐1 can regulate the PD‐L1 distribution at the transcriptional level. Herein, we tried to focus on the most important regulatory mechanisms of PD‐L1 by inducible agents in the tumor cells, such as signaling pathways, transcriptional factors, and posttranscriptional factors. Finally, these approaches may open up new windows for targeting tumor immune evasion and suggest the novel suppressors of PD‐L1 for efficient therapeutics.
中文翻译:

肿瘤微环境在PD-L1调控中的作用:对癌症免疫治疗的抗性中的新作用。
从宿主免疫系统逃避肿瘤是肿瘤发展和生存的重要策略。癌细胞中许多免疫检查点蛋白的表达是肿瘤细胞逃离免疫系统的一种机制。可以极大地影响肿瘤发展和癌症治疗的著名免疫检查点包括程序性死亡配体-1 /程序性死亡-1(PD-L1 / PD-1)。为了解决此现象并改善癌症治疗的治疗策略,以PD-L1 / PD-1途径的阻滞为靶点,但PD L1 / PD-1阻滞的治疗优势并未达到预期。在许多患者中,这种状况可能与不同类型的耐药性相关。克服对免疫检查点封锁疗法的抗性的关键问题是了解如何调节PD-L1水平。但是,调节PD-L1表达的机制很复杂,并且它们可能以不同的水平发生,从信号传导途径到转录后水平。例如,各种转录因子,例如缺氧诱导因子-1,核因子κΒ,干扰素γ,STAT3,MYC和AP-1,可以在转录水平上调节PD-L1的分布。在本文中,我们尝试着眼于肿瘤细胞中可诱导因子对PD-L1的最重要调节机制,例如信号传导途径,转录因子和转录后因子。最后,
更新日期:2020-04-02
中文翻译:

肿瘤微环境在PD-L1调控中的作用:对癌症免疫治疗的抗性中的新作用。
从宿主免疫系统逃避肿瘤是肿瘤发展和生存的重要策略。癌细胞中许多免疫检查点蛋白的表达是肿瘤细胞逃离免疫系统的一种机制。可以极大地影响肿瘤发展和癌症治疗的著名免疫检查点包括程序性死亡配体-1 /程序性死亡-1(PD-L1 / PD-1)。为了解决此现象并改善癌症治疗的治疗策略,以PD-L1 / PD-1途径的阻滞为靶点,但PD L1 / PD-1阻滞的治疗优势并未达到预期。在许多患者中,这种状况可能与不同类型的耐药性相关。克服对免疫检查点封锁疗法的抗性的关键问题是了解如何调节PD-L1水平。但是,调节PD-L1表达的机制很复杂,并且它们可能以不同的水平发生,从信号传导途径到转录后水平。例如,各种转录因子,例如缺氧诱导因子-1,核因子κΒ,干扰素γ,STAT3,MYC和AP-1,可以在转录水平上调节PD-L1的分布。在本文中,我们尝试着眼于肿瘤细胞中可诱导因子对PD-L1的最重要调节机制,例如信号传导途径,转录因子和转录后因子。最后,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号