当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of nitrocatechol chalcone and pyrazoline derivatives as inhibitors of catechol-O-methyltransferase and monoamine oxidase.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-04-11 , DOI: 10.1016/j.bmcl.2020.127188
Rialette Hitge 1 , Sharissa Smit 1 , Anél Petzer 1 , Jacobus P Petzer 1
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-04-11 , DOI: 10.1016/j.bmcl.2020.127188
Rialette Hitge 1 , Sharissa Smit 1 , Anél Petzer 1 , Jacobus P Petzer 1
Affiliation
|
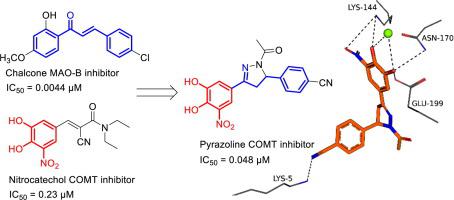
Literature reports that chalcones inhibit the monoamine oxidase (MAO) enzymes, mostly with specificity for the MAO-B isoform, while nitrocatechol compounds are established inhibitors of catechol-O-methyltransferase (COMT). Based on this, nitrocatechol derivatives of chalcone have been proposed to represent dual-target-directed compounds that may inhibit both MAO-B and COMT. Both these enzymes play key roles in the metabolism of dopamine and levodopa, and inhibitors are thus relevant to the treatment of Parkinson's disease. The present study expands on the discovery of dual MAO-B/COMT inhibitors by synthesising additional nitrocatechol derivatives of chalcones which include heterocyclic derivatives, and converting them to the corresponding pyrazoline derivatives. The newly synthesised chalcone and pyrazoline compounds were evaluated as inhibitors of human MAO and rat COMT, and the inhibition potencies were expressed as IC50 values. A pyrazoline derivative, compound 8b, was the most potent COMT inhibitor with an IC50 value of 0.048 μM. This is more potent than the reference COMT inhibitor, entacapone, which has an IC50 value of 0.23 μM. The results indicated that the pyrazoline derivatives (IC50 = 0.048-0.21 µM) are more potent COMT inhibitors than the chalcones (IC50 = 0.14-0.29 µM). Unfortunately, the chalcone and pyrazoline derivatives were weak MAO inhibitors with IC50 values > 41.4 µM. This study concludes that the nitrocatechol derivatives investigated here are promising COMT inhibitors, while not being suitable as MAO inhibitors. Using molecular docking, potential binding modes and interactions of selected inhibitors with COMT are proposed.
中文翻译:

评价作为邻苯二酚-O-甲基转移酶和单胺氧化酶抑制剂的硝基儿茶酚查尔酮和吡唑啉衍生物。
文献报道,查耳酮可抑制单胺氧化酶(MAO)酶,大多对MAO-B同工型具有特异性,而硝基儿茶酚化合物已被确立为儿茶酚-O-甲基转移酶(COMT)的抑制剂。基于此,提出了查尔酮的硝基邻苯二酚衍生物代表可同时抑制MAO-B和COMT的双靶标定向化合物。这两种酶均在多巴胺和左旋多巴的代谢中起关键作用,因此抑制剂与帕金森氏病的治疗有关。本研究扩展了双重MAO-B / COMT抑制剂的发现,方法是合成包括杂环衍生物在内的查耳酮的其他硝基邻苯二酚衍生物,并将其转化为相应的吡唑啉衍生物。评价新合成的查耳酮和吡唑啉化合物作为人MAO和大鼠COMT的抑制剂,并将抑制能力表示为IC 50值。吡唑啉衍生物化合物8b是最有效的COMT抑制剂,IC50值为0.048μM。这比参考COMT抑制剂entacapone更有效,后者的IC50值为0.23μM。结果表明,吡唑啉衍生物(IC50 = 0.048-0.21 µM)是比查耳酮(IC50 = 0.14-0.29 µM)更有效的COMT抑制剂。不幸的是,查尔酮和吡唑啉衍生物是较弱的MAO抑制剂,IC50值> 41.4 µM。这项研究得出的结论是,本文研究的硝基邻苯二酚衍生物是有希望的COMT抑制剂,但不适合用作MAO抑制剂。使用分子对接
更新日期:2020-04-11
中文翻译:

评价作为邻苯二酚-O-甲基转移酶和单胺氧化酶抑制剂的硝基儿茶酚查尔酮和吡唑啉衍生物。
文献报道,查耳酮可抑制单胺氧化酶(MAO)酶,大多对MAO-B同工型具有特异性,而硝基儿茶酚化合物已被确立为儿茶酚-O-甲基转移酶(COMT)的抑制剂。基于此,提出了查尔酮的硝基邻苯二酚衍生物代表可同时抑制MAO-B和COMT的双靶标定向化合物。这两种酶均在多巴胺和左旋多巴的代谢中起关键作用,因此抑制剂与帕金森氏病的治疗有关。本研究扩展了双重MAO-B / COMT抑制剂的发现,方法是合成包括杂环衍生物在内的查耳酮的其他硝基邻苯二酚衍生物,并将其转化为相应的吡唑啉衍生物。评价新合成的查耳酮和吡唑啉化合物作为人MAO和大鼠COMT的抑制剂,并将抑制能力表示为IC 50值。吡唑啉衍生物化合物8b是最有效的COMT抑制剂,IC50值为0.048μM。这比参考COMT抑制剂entacapone更有效,后者的IC50值为0.23μM。结果表明,吡唑啉衍生物(IC50 = 0.048-0.21 µM)是比查耳酮(IC50 = 0.14-0.29 µM)更有效的COMT抑制剂。不幸的是,查尔酮和吡唑啉衍生物是较弱的MAO抑制剂,IC50值> 41.4 µM。这项研究得出的结论是,本文研究的硝基邻苯二酚衍生物是有希望的COMT抑制剂,但不适合用作MAO抑制剂。使用分子对接

































 京公网安备 11010802027423号
京公网安备 11010802027423号