当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Reversible Cuprous Mediated Cathode Chemistry for Magnesium Batteries.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-04-11 , DOI: 10.1002/anie.202002177 Xiangyang Cheng,Zhonghua Zhang,Qingyu Kong,Qinghua Zhang,Tao Wang,Shanmu Dong,Lin Gu,Xiao Wang,Jun Ma,Pengxian Han,Hong-Ji Lin,Chien-Te Chen,Guanglei Cui
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-04-11 , DOI: 10.1002/anie.202002177 Xiangyang Cheng,Zhonghua Zhang,Qingyu Kong,Qinghua Zhang,Tao Wang,Shanmu Dong,Lin Gu,Xiao Wang,Jun Ma,Pengxian Han,Hong-Ji Lin,Chien-Te Chen,Guanglei Cui
|
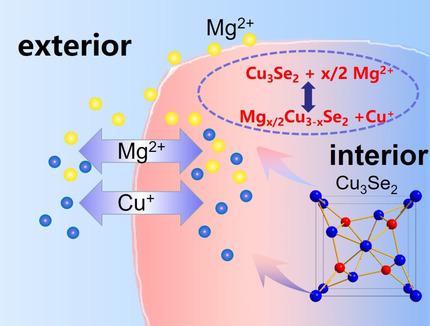
Sluggish kinetics and poor reversibility of cathode chemistry is the major challenge for magnesium batteries to achieve high volumetric capacity. Introduction of the cuprous ion (Cu+) as a charge carrier can decouple the magnesiation related energy storage from the cathode electrochemistry. Cu+ is generated from a fast equilibrium between copper selenide electrode and Mg electrolyte during standing time, rather than in the electrochemical process. A reversible chemical magnesiation/de‐magnesiation can be driven by this solid/liquid equilibrium. During a typical discharge process, Cu+ is reduced to Cu and drives the equilibrium to promote the magnesiation process. The reversible Cu to Cu+ redox promotes the recharge process. This novel Cu+ mediated cathode chemistry of Mg battery leads to a high reversible areal capacity of 12.5 mAh cm−2 with high mass loading (49.1 mg cm−2) of the electrode. 80 % capacity retention can be achieved for 200 cycles after a conditioning process.
中文翻译:

用于镁电池的高度可逆的亚铜介导的阴极化学。
动力学缓慢和阴极化学的可逆性差是镁电池实现高容量的主要挑战。引入亚铜离子(Cu +)作为电荷载体可以使与放大倍数相关的能量存储与阴极电化学分离。Cu +是由硒化铜电极和Mg电解质在静置时间内而不是在电化学过程中快速平衡产生的。这种固/液平衡可以驱动可逆的化学放大/缩小。在典型的放电过程中,Cu +还原为Cu并推动平衡,从而促进了放大过程。可逆的铜到铜+氧化还原促进充电过程。Mg电池的这种新颖的Cu +介导的阴极化学性质导致电极的高质量负载(49.1 mg cm -2)的高可逆面容量为12.5 mAh cm -2。调理过程完成200个循环后,可获得80%的容量保持率。
更新日期:2020-04-11
中文翻译:

用于镁电池的高度可逆的亚铜介导的阴极化学。
动力学缓慢和阴极化学的可逆性差是镁电池实现高容量的主要挑战。引入亚铜离子(Cu +)作为电荷载体可以使与放大倍数相关的能量存储与阴极电化学分离。Cu +是由硒化铜电极和Mg电解质在静置时间内而不是在电化学过程中快速平衡产生的。这种固/液平衡可以驱动可逆的化学放大/缩小。在典型的放电过程中,Cu +还原为Cu并推动平衡,从而促进了放大过程。可逆的铜到铜+氧化还原促进充电过程。Mg电池的这种新颖的Cu +介导的阴极化学性质导致电极的高质量负载(49.1 mg cm -2)的高可逆面容量为12.5 mAh cm -2。调理过程完成200个循环后,可获得80%的容量保持率。































 京公网安备 11010802027423号
京公网安备 11010802027423号