当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Myofibrillar protein conformation enhance gel properties of mixed surimi gels with Nemipterus virgatus and Hypophthalmichthys molitrix
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.foodhyd.2020.105924 Shumin Yi , Qiang Li , Cuiping Qiao , Chang Zhang , Wei Wang , Yongxia Xu , Hongbo Mi , Xuepeng Li , Jianrong Li
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.foodhyd.2020.105924 Shumin Yi , Qiang Li , Cuiping Qiao , Chang Zhang , Wei Wang , Yongxia Xu , Hongbo Mi , Xuepeng Li , Jianrong Li
|
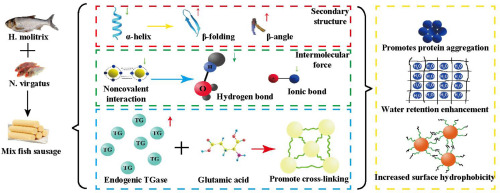
Abstract Marine and freshwater fish surimi exhibit a synergistic effect when mixed in a suitable ratio. In this paper, we investigated changing in myofibrillar protein conformation enhance the properties of mixed surimi gels formed by thermal processing. The mixtures of Nemipterus virgatus and Hypophthalmichthys molitrix surimi were prepared (N:H mass ratios of 0:1, 1:0, 3:1, and 1:5) and analyzed during thermal processing. In particular, changes in gel properties, microstructure, and protein conformation during gel formation were studied. The obtained results show that heating increases the fracture constant of the 3:1 and 1:5 surimi mixtures from 143.64 N/m and 68.40 N/m to 442.78 N/m and 320.99 N/m, respectively, and the fracture constant of the 1:0 and 0:1 surimi from 87.07 N/m and 56.71 N/m to 443.18 N/m and 237.42 N/m, respectively. Similarly, the water holding capacities, whiteness values, β-sheet, β-turn, and disulfide bond contents of these two gels increase with increasing processing time at 40 then 90 °C. Meanwhile, nonspecific association, hydrogen bonding, and α-helix contents in the N:H = 3:1 and N:H = 1:5 samples decrease under the effect of thermal processing. Microstructural images show that the network structure of the mixed surimi gel is tighter and more compact than that of the single-component gels. Based on these results, the synergistic effect is induced by the interactions between proteins from different sources in a suitable ratio.
中文翻译:

肌原纤维蛋白构象增强混合鱼糜凝胶与 Nemipterus virgatus 和 Hypophthalmichthys molitrix 的凝胶特性
摘要 海、淡水鱼鱼糜以合适的比例混合,具有协同增效作用。在本文中,我们研究了肌原纤维蛋白构象的变化增强了通过热处理形成的混合鱼糜凝胶的特性。制备了 Nemipterus virgatus 和 Hypophthalmichthys molitrix 鱼糜的混合物(N:H 质量比为 0:1、1:0、3:1 和 1:5),并在热处理过程中进行分析。特别是,研究了凝胶形成过程中凝胶特性、微观结构和蛋白质构象的变化。所得结果表明,加热使 3:1 和 1:5 鱼糜混合物的断裂常数分别从 143.64 N/m 和 68.40 N/m 增加到 442.78 N/m 和 320.99 N/m,以及1:0 和 0:1 鱼糜分别从 87.07 N/m 和 56.71 N/m 到 443.18 N/m 和 237.42 N/m。类似地,这两种凝胶的持水能力、白度值、β-折叠、β-转角和二硫键含量随着在 40 和 90 °C 下处理时间的增加而增加。同时,在热处理的影响下,N:H = 3:1 和 N:H = 1:5 样品中的非特异性缔合、氢键和α-螺旋含量降低。显微结构图像表明,混合鱼糜凝胶的网络结构比单组分凝胶更紧密、更致密。基于这些结果,协同效应是由不同来源的蛋白质以合适的比例相互作用引起的。在热处理的影响下,N:H = 3:1 和 N:H = 1:5 样品中的α-螺旋含量减少。微观结构图像表明,混合鱼糜凝胶的网络结构比单组分凝胶更紧密、更致密。基于这些结果,协同效应是由不同来源的蛋白质以合适的比例相互作用引起的。在热处理的影响下,N:H = 3:1 和 N:H = 1:5 样品中的α-螺旋含量减少。显微结构图像表明,混合鱼糜凝胶的网络结构比单组分凝胶更紧密、更致密。基于这些结果,协同效应是由不同来源的蛋白质以合适的比例相互作用引起的。
更新日期:2020-09-01
中文翻译:

肌原纤维蛋白构象增强混合鱼糜凝胶与 Nemipterus virgatus 和 Hypophthalmichthys molitrix 的凝胶特性
摘要 海、淡水鱼鱼糜以合适的比例混合,具有协同增效作用。在本文中,我们研究了肌原纤维蛋白构象的变化增强了通过热处理形成的混合鱼糜凝胶的特性。制备了 Nemipterus virgatus 和 Hypophthalmichthys molitrix 鱼糜的混合物(N:H 质量比为 0:1、1:0、3:1 和 1:5),并在热处理过程中进行分析。特别是,研究了凝胶形成过程中凝胶特性、微观结构和蛋白质构象的变化。所得结果表明,加热使 3:1 和 1:5 鱼糜混合物的断裂常数分别从 143.64 N/m 和 68.40 N/m 增加到 442.78 N/m 和 320.99 N/m,以及1:0 和 0:1 鱼糜分别从 87.07 N/m 和 56.71 N/m 到 443.18 N/m 和 237.42 N/m。类似地,这两种凝胶的持水能力、白度值、β-折叠、β-转角和二硫键含量随着在 40 和 90 °C 下处理时间的增加而增加。同时,在热处理的影响下,N:H = 3:1 和 N:H = 1:5 样品中的非特异性缔合、氢键和α-螺旋含量降低。显微结构图像表明,混合鱼糜凝胶的网络结构比单组分凝胶更紧密、更致密。基于这些结果,协同效应是由不同来源的蛋白质以合适的比例相互作用引起的。在热处理的影响下,N:H = 3:1 和 N:H = 1:5 样品中的α-螺旋含量减少。微观结构图像表明,混合鱼糜凝胶的网络结构比单组分凝胶更紧密、更致密。基于这些结果,协同效应是由不同来源的蛋白质以合适的比例相互作用引起的。在热处理的影响下,N:H = 3:1 和 N:H = 1:5 样品中的α-螺旋含量减少。显微结构图像表明,混合鱼糜凝胶的网络结构比单组分凝胶更紧密、更致密。基于这些结果,协同效应是由不同来源的蛋白质以合适的比例相互作用引起的。































 京公网安备 11010802027423号
京公网安备 11010802027423号