当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibria of the Quaternary System (Li2SO4 + Na2SO4 + MgSO4 + H2O) at 288.15 K: Experimental and Model Simulation
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-04-10 , DOI: 10.1021/acs.jced.0c00003 Shiqiang Wang 1, 2 , Peng Gu 1 , Fei Yuan 1, 2 , Xunian Han 1 , Yafei Guo 1, 2 , Tianlong Deng 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-04-10 , DOI: 10.1021/acs.jced.0c00003 Shiqiang Wang 1, 2 , Peng Gu 1 , Fei Yuan 1, 2 , Xunian Han 1 , Yafei Guo 1, 2 , Tianlong Deng 1, 2
Affiliation
|
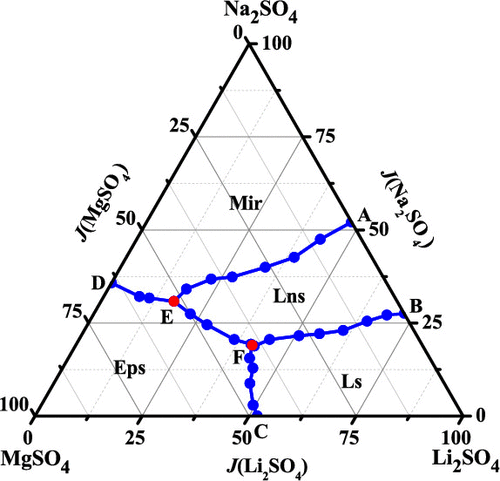
Solubility data for the quaternary system (Li2SO4 + Na2SO4 + MgSO4 + H2O) is very important for the separation of lithium sulfate from the salt lake brines in Qaidam Basin. The experimental and calculated solubilities of the quaternary system (Li2SO4 + Na2SO4 + MgSO4 + H2O) at T = 288.15 K and P = 0.1 MPa were determined. It is shown the solid phases Li2SO4·H2O, Na2SO4·10H2O, and MgSO4·7H2O and a new complex salt Li2SO4·3Na2SO4·12H2O exist in this system. The solubilities of this quaternary system were predicted using the Harvie–Møller–Weare (HMW) model of electrolyte ion-interaction theory. The modeling solubility isotherms agree well with experimental data, which indicates the solubilities and model parameters obtained in this work are reliable.
中文翻译:

288.15 K时四元体系(Li 2 SO 4 + Na 2 SO 4 + MgSO 4 + H 2 O)的固液相平衡:实验和模型模拟
四元系统的溶解度数据(Li 2 SO 4 + Na 2 SO 4 + MgSO 4 + H 2 O)对于从柴达木盆地盐湖盐水中分离硫酸锂非常重要。确定了在T = 288.15 K和P = 0.1 MPa时四元体系(Li 2 SO 4 + Na 2 SO 4 + MgSO 4 + H 2 O)的实验和计算溶解度。示出了固相Li 2 SO 4 ·H 2 O,Na 2 SO4 ·10H 2 O,并用MgSO 4 ·7H 2 O和新的配盐栗2 SO 4 ·3NA 2 SO 4 ·12H 2 ö在该系统中存在。使用电解质离子相互作用理论的Harvie-Møller-Weare(HMW)模型预测了该四元体系的溶解度。建模的溶解度等温线与实验数据吻合良好,表明在这项工作中获得的溶解度和模型参数是可靠的。
更新日期:2020-04-10
中文翻译:

288.15 K时四元体系(Li 2 SO 4 + Na 2 SO 4 + MgSO 4 + H 2 O)的固液相平衡:实验和模型模拟
四元系统的溶解度数据(Li 2 SO 4 + Na 2 SO 4 + MgSO 4 + H 2 O)对于从柴达木盆地盐湖盐水中分离硫酸锂非常重要。确定了在T = 288.15 K和P = 0.1 MPa时四元体系(Li 2 SO 4 + Na 2 SO 4 + MgSO 4 + H 2 O)的实验和计算溶解度。示出了固相Li 2 SO 4 ·H 2 O,Na 2 SO4 ·10H 2 O,并用MgSO 4 ·7H 2 O和新的配盐栗2 SO 4 ·3NA 2 SO 4 ·12H 2 ö在该系统中存在。使用电解质离子相互作用理论的Harvie-Møller-Weare(HMW)模型预测了该四元体系的溶解度。建模的溶解度等温线与实验数据吻合良好,表明在这项工作中获得的溶解度和模型参数是可靠的。































 京公网安备 11010802027423号
京公网安备 11010802027423号