当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Positive Effects of H2O on the Hydrogen Oxidation Reaction on Sr2Fe1.5Mo0.5O6−δ-Based Perovskite Anodes for Solid Oxide Fuel Cells
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-04-10 , DOI: 10.1021/acscatal.9b05458
He Qi 1 , Yueh-Lin Lee 2, 3 , Tao Yang 4, 5 , Wenyuan Li 1 , Wei Li 1 , Liang Ma 1, 6 , Shanshan Hu 1 , Yuhua Duan 2 , Gregory A. Hackett 4 , Xingbo Liu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-04-10 , DOI: 10.1021/acscatal.9b05458
He Qi 1 , Yueh-Lin Lee 2, 3 , Tao Yang 4, 5 , Wenyuan Li 1 , Wei Li 1 , Liang Ma 1, 6 , Shanshan Hu 1 , Yuhua Duan 2 , Gregory A. Hackett 4 , Xingbo Liu 1
Affiliation
|
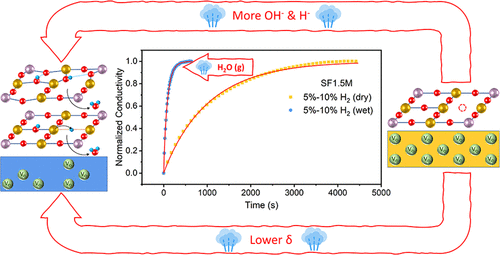
The steam is commonly supplied with H2 to the anodes of Ni-based solid oxide fuel cells (SOFCs). Humidified H2 is also widely used for emerging perovskite anodes of SOFCs, although the influences of H2O on the hydrogen oxidation reaction (HOR) on their surfaces have not been well understood yet. In this work, the effects of H2O on the HOR on Sr2Fe1.5Mo0.5O6−δ (SF1.5M), La0.5Sr1.5Fe1.5Mo0.5O6−δ (LSFM), and Pr0.5Sr1.5Fe1.5Mo0.5O6−δ (PSFM) were systematically investigated using the electrochemical impedance spectroscopy (EIS) and electrical conductivity relaxation (ECR) methods. The EIS spectra suggested the possible promotional effect of H2O on decreasing the polarization resistance of SF1.5M. The ECR results confirmed that the presence of H2O in H2 can significantly increase the oxygen exchange coefficients of SF1.5M, LSFM, and PSFM. To gain a mechanistic understanding of the role of H2O, the density functional theory-based calculations and thermodynamic modeling were performed to unravel the effect of H2O on the HOR on the SF1.5M (001) surfaces. Benefiting from the increment of the oxygen chemical potential and decrement of the free electron concentration upon increasing humidity, the plateau intermediate state in the HOR energy landscape, the step of H2O plus surface oxygen vacancy formation, is reduced on the SF1.5M (001) BO2 (B = Fe and Mo)-terminated surfaces, when decreasing the slab oxygen nonstoichiometry. Furthermore, by comparing the scenarios of the HOR on the dry and hydrated surfaces, the H2O plus surface oxygen vacancy formation energies are lower in the latter case. These two proposed factors, i.e., (i) change of electron chemical potential upon the change of near-surface δ and (ii) enhanced interaction of surface H species with the hydrated perovskite surfaces, contribute to the promoted HOR in the presence of H2O. This work provides important insights into the effects of H2O on the HOR for SOFCs.
中文翻译:

H的正效应2 O于上锶的氢的氧化反应2的Fe 1.5沫0.5 ø 6-δ系钙钛矿型阳极的固体氧化物燃料电池
蒸汽通常与H 2一起提供给Ni基固体氧化物燃料电池(SOFC)的阳极。加湿的H 2还广泛用于新兴的SOFC钙钛矿阳极,尽管H 2 O对其表面氢氧化反应(HOR)的影响尚未充分了解。在这项工作中,H的影响2 O于上锶的HOR 2的Fe 1.5沫0.5 ø 6-δ(SF1.5M),LA 0.5锶1.5铁1.5沫0.5 ø 6-δ(LSFM),和Pr 0.5锶1.5铁1.5钼0.5 ø 6-δ(PSFM)使用电化学阻抗谱(EIS)和导电性松弛(ECR)方法进行了系统的研究。EIS光谱表明,H 2 O可能会降低SF1.5M的极化电阻。ECR结果证实H 2 O在H 2中的存在可以显着增加SF1.5M,LSFM和PSFM的氧交换系数。为了从机械角度了解H 2 O的作用,进行了基于密度泛函理论的计算和热力学建模,以揭示H 2的作用。SF1.5M(001)表面上的HOR上的O。得益于氧气化学势的增加和湿度的增加,自由电子浓度的下降,在SF1.5M上,HOR能量格局中的高原中间态,H 2 O加表面氧空位形成的步骤得以减少( 001)当降低平板氧气非化学计量比时,以BO 2(B = Fe和Mo)为末端的表面。此外,通过比较干燥和水合表面上的HOR情景,H 2在后一种情况下,O加表面氧空位形成能较低。提出的这两个因素,即(i)随着近表面δ的变化电子化学势的变化和(ii)表面H物种与水合钙钛矿表面的相互作用增强,在存在H 2时促进了HOR的产生。O.这项工作为H 2 O对SOFC的HOR的影响提供了重要的见识。
更新日期:2020-04-10
中文翻译:

H的正效应2 O于上锶的氢的氧化反应2的Fe 1.5沫0.5 ø 6-δ系钙钛矿型阳极的固体氧化物燃料电池
蒸汽通常与H 2一起提供给Ni基固体氧化物燃料电池(SOFC)的阳极。加湿的H 2还广泛用于新兴的SOFC钙钛矿阳极,尽管H 2 O对其表面氢氧化反应(HOR)的影响尚未充分了解。在这项工作中,H的影响2 O于上锶的HOR 2的Fe 1.5沫0.5 ø 6-δ(SF1.5M),LA 0.5锶1.5铁1.5沫0.5 ø 6-δ(LSFM),和Pr 0.5锶1.5铁1.5钼0.5 ø 6-δ(PSFM)使用电化学阻抗谱(EIS)和导电性松弛(ECR)方法进行了系统的研究。EIS光谱表明,H 2 O可能会降低SF1.5M的极化电阻。ECR结果证实H 2 O在H 2中的存在可以显着增加SF1.5M,LSFM和PSFM的氧交换系数。为了从机械角度了解H 2 O的作用,进行了基于密度泛函理论的计算和热力学建模,以揭示H 2的作用。SF1.5M(001)表面上的HOR上的O。得益于氧气化学势的增加和湿度的增加,自由电子浓度的下降,在SF1.5M上,HOR能量格局中的高原中间态,H 2 O加表面氧空位形成的步骤得以减少( 001)当降低平板氧气非化学计量比时,以BO 2(B = Fe和Mo)为末端的表面。此外,通过比较干燥和水合表面上的HOR情景,H 2在后一种情况下,O加表面氧空位形成能较低。提出的这两个因素,即(i)随着近表面δ的变化电子化学势的变化和(ii)表面H物种与水合钙钛矿表面的相互作用增强,在存在H 2时促进了HOR的产生。O.这项工作为H 2 O对SOFC的HOR的影响提供了重要的见识。

































 京公网安备 11010802027423号
京公网安备 11010802027423号