当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2‐(2‐Hydroxyaryl)‐4H‐benzo[e][1,3]oxazin‐4‐ones by Palladium‐Catalyzed C(sp2)−H Hydroxylation via Electro‐chemical Oxidation
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-09 , DOI: 10.1002/adsc.202000173 Hongfeng Wu 1, 2 , Qi An 1, 2 , Chaoyin He 1, 2 , Xiaodong Fan 1, 2 , Weihao Guo 1, 2 , Minghui Zuo 1, 2 , Chunzhao Xu 1, 2 , Rui Guo 1, 2 , Wenyi Chu 1, 2 , Zhizhong Sun 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-09 , DOI: 10.1002/adsc.202000173 Hongfeng Wu 1, 2 , Qi An 1, 2 , Chaoyin He 1, 2 , Xiaodong Fan 1, 2 , Weihao Guo 1, 2 , Minghui Zuo 1, 2 , Chunzhao Xu 1, 2 , Rui Guo 1, 2 , Wenyi Chu 1, 2 , Zhizhong Sun 1, 2
Affiliation
|
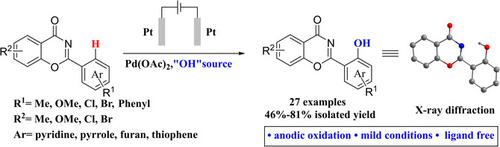
An electrochemical direct ortho ‐hydroxylation of 2‐aryl‐4H ‐benzo[e ][1,3]oxazin‐4‐ones was developed with Pd(OAc)2 as catalyst, oxazine ring as a directing group and Oxone as the hydroxylation reagent. A series of hydroxylation products were obtained under mild conditions, and the yields were from medium to good. This method is characterized by good functional group tolerance and a wide range of substrates. More importantly, use anodic oxidation to avoid the use of potentially toxic and polluting oxidants. A gram‐scale direct electrochemical hydroxylation of 2‐phenyl‐4H ‐benzo[e ][1,3]oxazin‐4‐one was performed, and the hydroxylation product was applied to synthesize the drug deferasirox. In addition, the single crystal of 2‐(2‐hydroxyphenyl)‐4H ‐benzo[e ][1,3]oxazin‐4‐one was obtained and determined by X‐ray diffraction. Finally, the reaction mechanism was proposed and verified by cyclic voltammetry (CV). This protocol also provides an alternative electrochemical hydroxylation methodology for the functionalization of molecules.
中文翻译:

钯催化C(sp2)-H的电化学氧化羟化反应合成2-(2-羟基芳基)-4H-苯并[e] [1,3]恶嗪-4-酮
以Pd(OAc)2为催化剂,恶嗪环为导向基团和Oxone为羟基化作用开发了2-芳基-4 H-苯并[ e ] [1,3]恶嗪-4-酮的电化学直接邻羟基化反应试剂。在温和条件下获得了一系列羟基化产物,收率从中等到良好。该方法的特征在于良好的官能团耐受性和广泛的底物。更重要的是,使用阳极氧化以避免使用潜在的有毒和污染性氧化剂。的2-苯基-4-甲克级直接电化学羟化ħ苯并[ ë进行] [1,3]恶嗪-4-酮的合成,并应用羟基化产物合成地拉罗司。另外,获得了2-(2-羟基苯基)-4- H-苯并[ e ] [1,3]恶嗪-4-酮的单晶并通过X射线衍射测定。最后,提出了反应机理并通过循环伏安法(CV)进行了验证。该协议还提供了分子功能化的另一种电化学羟基化方法。
更新日期:2020-04-09
中文翻译:

钯催化C(sp2)-H的电化学氧化羟化反应合成2-(2-羟基芳基)-4H-苯并[e] [1,3]恶嗪-4-酮
以Pd(OAc)2为催化剂,恶嗪环为导向基团和Oxone为羟基化作用开发了2-芳基-4 H-苯并[ e ] [1,3]恶嗪-4-酮的电化学直接邻羟基化反应试剂。在温和条件下获得了一系列羟基化产物,收率从中等到良好。该方法的特征在于良好的官能团耐受性和广泛的底物。更重要的是,使用阳极氧化以避免使用潜在的有毒和污染性氧化剂。的2-苯基-4-甲克级直接电化学羟化ħ苯并[ ë进行] [1,3]恶嗪-4-酮的合成,并应用羟基化产物合成地拉罗司。另外,获得了2-(2-羟基苯基)-4- H-苯并[ e ] [1,3]恶嗪-4-酮的单晶并通过X射线衍射测定。最后,提出了反应机理并通过循环伏安法(CV)进行了验证。该协议还提供了分子功能化的另一种电化学羟基化方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号