Nature Communications ( IF 14.7 ) Pub Date : 2020-04-09 , DOI: 10.1038/s41467-020-15577-2
Satoru Torii 1 , Hirofumi Yamaguchi 1 , Akira Nakanishi 2 , Satoko Arakawa 1 , Shinya Honda 1 , Kenta Moriwaki 3 , Hiroyasu Nakano 4 , Shigeomi Shimizu 1
|
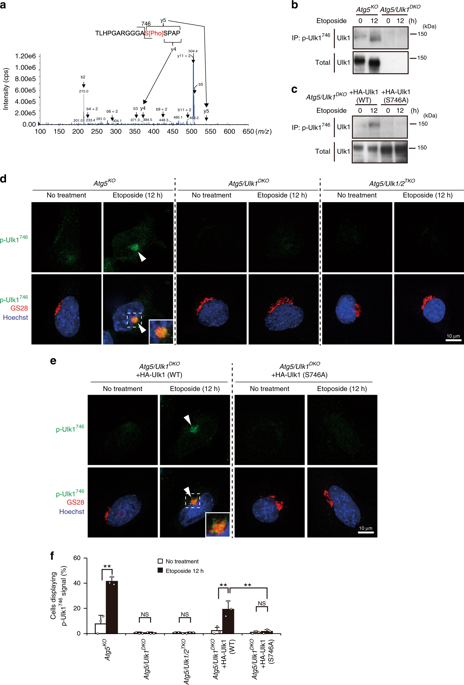
Alternative autophagy is an autophagy-related protein 5 (Atg5)-independent type of macroautophagy. Unc51-like kinase 1 (Ulk1) is an essential initiator not only for Atg5-dependent canonical autophagy but also for alternative autophagy. However, the mechanism as to how Ulk1 differentially regulates both types of autophagy has remained unclear. In this study, we identify a phosphorylation site of Ulk1 at Ser746, which is phosphorylated during genotoxic stress-induced alternative autophagy. Phospho-Ulk1746 localizes exclusively on the Golgi and is required for alternative autophagy, but not canonical autophagy. We also identify receptor-interacting protein kinase 3 (RIPK3) as the kinase responsible for genotoxic stress-induced Ulk1746 phosphorylation, because RIPK3 interacts with and phosphorylates Ulk1 at Ser746, and loss of RIPK3 abolishes Ulk1746 phosphorylation. These findings indicate that RIPK3-dependent Ulk1746 phosphorylation on the Golgi plays a pivotal role in genotoxic stress-induced alternative autophagy.
中文翻译:

遗传毒性应激诱导的替代自噬所需的Ulk1磷酸化位点的鉴定。
替代性自噬是一种自噬相关蛋白5(Atg5)独立的巨自噬类型。Unc51样激酶1(Ulk1)不仅是依赖于Atg5的规范自噬的重要引发剂,也是替代自噬的重要引发剂。但是,关于Ulk1如何差异调节两种自噬的机制仍不清楚。在这项研究中,我们确定了Serk 746处Ulk1的磷酸化位点,在遗传毒性应激诱导的自噬过程中被磷酸化。Phospho-Ulk1 746仅定位在高尔基体上,是替代性自噬(而非规范性自噬)所必需的。我们还确定受体相互作用蛋白激酶3(RIPK3)作为负责基因毒性应激诱导的Ulk1 746的激酶磷酸化,因为RIPK3在Ser 746处与Ulk1相互作用并使之磷酸化,而RIPK3的缺失则消除了Ulk1 746的磷酸化。这些发现表明,高尔基体上依赖RIPK3的Ulk1 746磷酸化在遗传毒性应激诱导的自噬中起着关键作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号