当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of novel carbazole hybrids as promising antimicrobial agents
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-04-15 , DOI: 10.1002/cbdv.201900550
Mahamadhanif S Shaikh 1 , Balakumar Chandrasekaran 1, 2 , Mahesh B Palkar 1 , Ashish M Kanhed 1 , Afsana Kajee 1, 3 , Koleka P Mlisana 3 , Parvesh Singh 4 , Meenu Ghai 5 , Mavela Cleopus Mahlalela 1 , Rajshekhar Karpoormath 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-04-15 , DOI: 10.1002/cbdv.201900550
Mahamadhanif S Shaikh 1 , Balakumar Chandrasekaran 1, 2 , Mahesh B Palkar 1 , Ashish M Kanhed 1 , Afsana Kajee 1, 3 , Koleka P Mlisana 3 , Parvesh Singh 4 , Meenu Ghai 5 , Mavela Cleopus Mahlalela 1 , Rajshekhar Karpoormath 1
Affiliation
|
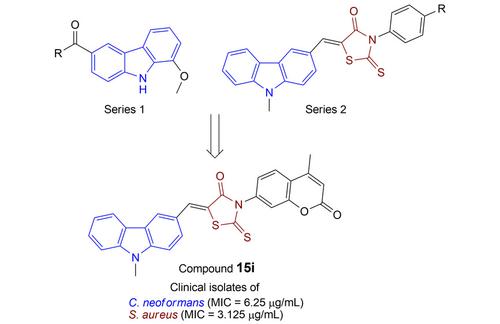
Two series of carbazole analogs of 8‐methoxy‐N‐substituted‐9H‐carbazole‐3‐carboxamides (series 1) and carbazolyl substituted rhodanines (series 2) were synthesized through facile synthetic routes. All the final compounds from these two series were evaluated for their preliminary in vitro antifungal and antibacterial activity against four fungal (Candida albicans, Cryptococcus neoformans, Cryptococcus tropicalis and Aspergillus niger) and four bacterial (Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa) strains, respectively. Among the tested compounds, three compounds of series 1 displayed promising antifungal and antibacterial activity, especially against C. neoformans and S. aureus. In addition, one compound of series 1 displayed notable antimicrobial activity (MIC: 6.25 μg/mL) against clinical isolates of C. albicans and C. neoformans (MIC: 12.5 μg/mL). From the second series, four compounds exhibited significant antifungal and antibacterial activity, especially against C. neoformans and S. aureus. The most active compound of series 2 displayed a prominent antimicrobial activity against C. neoformans (MIC: 3.125 μg/mL) and S. aureus (MIC: 1.56 μg/mL), respectively.
中文翻译:

作为有前景的抗菌剂的新型咔唑杂化物的合成和生物学评价
8-甲氧基-N-取代-9H-咔唑-3-甲酰胺(系列1)和咔唑基取代的若丹宁(系列2)的两个系列咔唑类似物通过简便的合成路线合成。评估了这两个系列的所有最终化合物对四种真菌(白色念珠菌、新型隐球菌、热带隐球菌和黑曲霉)和四种细菌(金黄色葡萄球菌、枯草芽孢杆菌、大肠杆菌和铜绿假单胞菌)的初步体外抗真菌和抗菌活性) 应变,分别。在测试的化合物中,系列 1 的三种化合物显示出有希望的抗真菌和抗菌活性,尤其是对新型隐球菌和金黄色葡萄球菌。此外,系列 1 的一种化合物显示出显着的抗微生物活性(MIC:6.25 μg/mL),对临床分离株 C. 白色念珠菌和新型隐球菌(MIC:12.5 μg/mL)。在第二个系列中,四种化合物表现出显着的抗真菌和抗菌活性,尤其是对新型隐球菌和金黄色葡萄球菌。系列 2 中活性最强的化合物分别显示出对新型隐球菌(MIC:3.125 μg/mL)和金黄色葡萄球菌(MIC:1.56 μg/mL)的显着抗菌活性。
更新日期:2020-04-15
中文翻译:

作为有前景的抗菌剂的新型咔唑杂化物的合成和生物学评价
8-甲氧基-N-取代-9H-咔唑-3-甲酰胺(系列1)和咔唑基取代的若丹宁(系列2)的两个系列咔唑类似物通过简便的合成路线合成。评估了这两个系列的所有最终化合物对四种真菌(白色念珠菌、新型隐球菌、热带隐球菌和黑曲霉)和四种细菌(金黄色葡萄球菌、枯草芽孢杆菌、大肠杆菌和铜绿假单胞菌)的初步体外抗真菌和抗菌活性) 应变,分别。在测试的化合物中,系列 1 的三种化合物显示出有希望的抗真菌和抗菌活性,尤其是对新型隐球菌和金黄色葡萄球菌。此外,系列 1 的一种化合物显示出显着的抗微生物活性(MIC:6.25 μg/mL),对临床分离株 C. 白色念珠菌和新型隐球菌(MIC:12.5 μg/mL)。在第二个系列中,四种化合物表现出显着的抗真菌和抗菌活性,尤其是对新型隐球菌和金黄色葡萄球菌。系列 2 中活性最强的化合物分别显示出对新型隐球菌(MIC:3.125 μg/mL)和金黄色葡萄球菌(MIC:1.56 μg/mL)的显着抗菌活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号