Archives of Cardiovascular Diseases ( IF 2.3 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.acvd.2020.01.003 Claire Arnaud 1 , Thomas Bochaton 2 , Jean-Louis Pépin 1 , Elise Belaidi 1
|
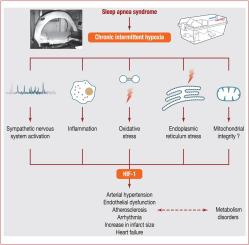
Obstructive sleep apnoea syndrome is a growing health concern, affecting nearly one billion people worldwide; it is an independent cardiovascular risk factor, associated with incident obesity, insulin resistance, hypertension, arrhythmias, stroke, coronary artery disease and heart failure. Obstructive sleep apnoea-related cardiovascular and metabolic co-morbidities are a major concern for prognosis and the complexity of obstructive sleep apnoea integrated care. Continuous positive airway pressure, the first-line therapy for the treatment of obstructive sleep apnoea, is highly effective at improving symptoms and quality of life, but has limited effect on co-morbidities. Deciphering the molecular pathways involved in obstructive sleep apnoea metabolic and cardiovascular consequences is a priority to make new pharmacological targets available, in combination with or as an alternative to continuous positive airway pressure. Intermittent hypoxia, a landmark feature of obstructive sleep apnoea, is the key intermediary mechanism underlying metabolic and cardiovascular complications. Experimental settings allowing intermittent hypoxia exposure in cells, rodents and healthy humans have been established to dissect the molecular mechanisms of obstructive sleep apnoea-related co-morbidities. The main objective of this review is to recapitulate the molecular pathways, cells and tissue interactions contributing to the cardiometabolic consequences of intermittent hypoxia. Sympathetic activation, low-grade inflammation, oxidative stress and endoplasmic reticulum stress are triggered by intermittent hypoxia and play a role in cardiometabolic dysfunction. The key role of hypoxia-inducible factor-1 transcription factor will be detailed, as well as the underestimated and less described importance of mitochondrial functional changes in the intermittent hypoxia setting.
中文翻译:

阻塞性睡眠呼吸暂停和心血管后果:病理生理机制。
阻塞性睡眠呼吸暂停综合症日益引起人们的健康关注,全世界有近十亿人受到影响。它是独立的心血管危险因素,与肥胖,胰岛素抵抗,高血压,心律不齐,中风,冠状动脉疾病和心力衰竭有关。阻塞性睡眠呼吸暂停相关的心血管和代谢合并症是阻塞性睡眠呼吸暂停综合护理的预后和复杂性的主要关注因素。持续的气道正压通气是阻塞性睡眠呼吸暂停的一线治疗,对改善症状和生活质量非常有效,但对合并症的影响有限。阐明阻塞性睡眠呼吸暂停代谢和心血管疾病后果所涉及的分子途径是提供新药理学目标的优先事项,与持续的气道正压结合或替代。间歇性缺氧是阻塞性睡眠呼吸暂停的标志性特征,是代谢和心血管并发症的关键中介机制。已经建立了允许在细胞,啮齿动物和健康人类中间歇性缺氧暴露的实验设置,以剖析阻塞性睡眠呼吸暂停相关合并症的分子机制。这篇综述的主要目的是概述间歇性缺氧对心脏代谢的影响的分子途径,细胞和组织相互作用。间歇性缺氧会触发交感神经激活,轻度炎症,氧化应激和内质网应激,并在心脏代谢功能异常中起作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号