Molecular Therapy - Nucleic Acids ( IF 6.5 ) Pub Date : 2020-04-07 , DOI: 10.1016/j.omtn.2020.03.016 Wenqi Sun 1 , Changyang Xing 2 , Lianbi Zhao 1 , Ping Zhao 2 , Guodong Yang 3 , Lijun Yuan 2
|
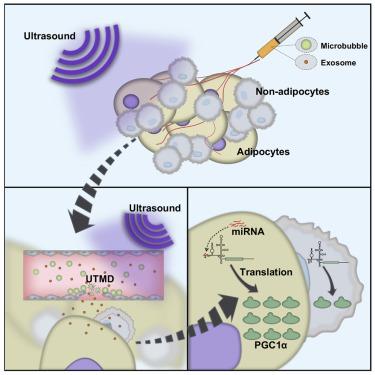
Exosome-mediated nucleic acids delivery has been emerging as a promising strategy for gene therapy. However, the intrinsic off-target effects due to non-specific uptake of exosomes by other tissues remain the big hurdle for clinical application. In this study, we aimed to enhance the efficacy and minimize the off-target effects by simultaneously encapsulating engineered mRNA translationally activated by tissue-specific microRNA (miRNA) and increasing targeted delivery efficiency via ultrasound-targeted microbubble destruction (UTMD). Briefly, the upstream of interest transcript was engineered to harbor an internal ribosome entry site (IRES) modified with two miRNA recognition sites. In vitro reporter experiments revealed that the engineered mRNA could be encapsulated into exosomes and can be translationally activated by corresponding miRNAs in the recipient cells. By a proof-of-principle in vivo experiment, we encapsulated miR-148a (an adipose relatively specific miRNA)-responsive PGC1α mRNA into exosomes and delivered the exosomes into the adipose tissue with the aid of UTMD. Efficient PGC1α translation was activated in the adipose tissue, together with obvious browning induction. Moreover, there was much lower off-target translation of PGC1 α in lungs and other tissues. Taken together, our study establishes a novel adipose-specific exosome delivery strategy to enhance efficacy and minimize off-target effects simultaneously.
中文翻译:

超声辅助组织反应性mRNA的外泌体递送,以提高功效和最小化脱靶效应。
外来体介导的核酸递送已经成为基因治疗的有前途的策略。然而,由于其他组织非特异性地吸收外来体而导致的固有脱靶效应仍然是临床应用的主要障碍。在这项研究中,我们旨在通过同时封装由组织特异性microRNA(miRNA)翻译激活的工程化mRNA,并通过超声靶向微泡破坏(UTMD)提高靶向递送效率,来提高功效并最小化脱靶效应。简而言之,将目的转录本的上游工程化,以容纳一个内部核糖体进入位点(IRES),该位点被两个miRNA识别位点修饰。体外记者实验表明,工程化的mRNA可以被封装到外泌体中,并且可以被受体细胞中相应的miRNA翻译激活。通过原则上的体内实验,我们将miR-148a(一种脂肪相对特定的miRNA)反应性PGC1αmRNA封装到囊泡中,并借助UTMD将其释放到脂肪组织中。在脂肪组织中有效的PGC1α翻译被激活,并具有明显的褐变诱导作用。而且,在肺和其他组织中PGC1α的脱靶翻译低得多。综上所述,我们的研究建立了一种新的脂肪特异性外泌体递送策略,以增强疗效并同时最小化脱靶效应。































 京公网安备 11010802027423号
京公网安备 11010802027423号