当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroxyl and sulfate radical-based oxidation of RhB dye in UV/H2O2 and UV/persulfate systems: Kinetics, mechanisms, and comparison.
Chemosphere ( IF 8.1 ) Pub Date : 2020-04-08 , DOI: 10.1016/j.chemosphere.2020.126655 Xinxin Ding 1 , Leonardo Gutierrez 2 , Jean-Philippe Croue 3 , Minrui Li 1 , Lijun Wang 1 , Yuru Wang 1
Chemosphere ( IF 8.1 ) Pub Date : 2020-04-08 , DOI: 10.1016/j.chemosphere.2020.126655 Xinxin Ding 1 , Leonardo Gutierrez 2 , Jean-Philippe Croue 3 , Minrui Li 1 , Lijun Wang 1 , Yuru Wang 1
Affiliation
|
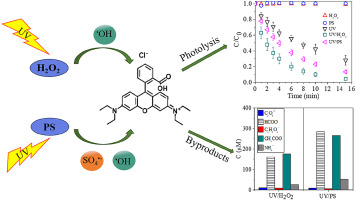
The degradation kinetics and mechanisms of Rhodamine B (RhB) dye by •OH and SO4•- based advanced oxidation processes were investigated. The •OH and SO4•- radicals were generated by UV photolysis of hydrogen peroxide and persulfate (i.e., UV/H2O2 and UV/PS), respectively. The effects of initial solution pH, RhB concentration, oxidant dosage, Fe2+ concentration, and water matrices were examined. The results showed that the degradation of RhB followed pseudo-first-order kinetics in both processes, with the UV/H2O2 process exhibiting better performance than that of the UV/PS process. Acidic conditions were favorable to the degradation of RhB in both systems. Increasing the oxidant dosage or decreasing the contaminant concentration could enhance the degradation of RhB. Photo-Fenton-like processes accelerated the performance when Fe2+ was added into both systems. The removal efficiency of RhB was inhibited upon the addition of humic substances. The addition of Cl- displayed no significant effect and promoted RhB degradation in UV/H2O2 and UV/PS systems, respectively. The presence of NO3- promoted RhB degradation, while H2PO4- and C2O42- showed an inhibitory effect on both UV/H2O2 and UV/PS processes. Radical scavenging tests revealed the dominant role of SO4•- radicals in the UV/PS system. Furthermore, the evolution of low molecular weight organic acids and NH4+ during the degradation of RhB in these two processes were compared. Both UV/H2O2 and UV/PS systems led to similar formation trends of NH4+ and some ring-opening products (e.g., formic acid, acetic acid, and oxalic acid), suggesting some analogies in the decay pathways of RhB by •OH and SO4•--induced oxidation processes.
中文翻译:

UV / H2O2和UV /过硫酸盐体系中RhB染料基于羟基和硫酸根的氧化:动力学,机理和比较。
研究了基于•OH和SO4•的高级氧化过程对若丹明B(RhB)染料的降解动力学及其机理。•OH和SO4•-自由基分别通过过氧化氢和过硫酸盐的UV光分解(即UV / H2O2和UV / PS)产生。检查了初始溶液pH,RhB浓度,氧化剂用量,Fe2 +浓度和水基质的影响。结果表明,RhB的降解在两个过程中均遵循伪一级动力学,其中UV / H2O2过程表现出比UV / PS过程更好的性能。酸性条件有利于两个系统中RhB的降解。增加氧化剂剂量或降低污染物浓度可以增强RhB的降解。当在两个系统中都添加Fe2 +时,类似光芬顿的工艺可提高性能。添加腐殖质会抑制RhB的去除效率。Cl-的添加在UV / H2O2和UV / PS系统中分别没有表现出明显的作用并促进RhB降解。NO3-的存在促进了RhB的降解,而H2PO4-和C2O42-则显示出对UV / H2O2和UV / PS过程的抑制作用。自由基清除测试表明SO4•-自由基在UV / PS系统中起主要作用。此外,比较了这两个过程中RhB降解过程中低分子量有机酸和NH4 +的演变。UV / H2O2和UV / PS系统都导致类似的NH4 +和一些开环产物(例如,甲酸,乙酸和草酸)的形成趋势,
更新日期:2020-04-08
中文翻译:

UV / H2O2和UV /过硫酸盐体系中RhB染料基于羟基和硫酸根的氧化:动力学,机理和比较。
研究了基于•OH和SO4•的高级氧化过程对若丹明B(RhB)染料的降解动力学及其机理。•OH和SO4•-自由基分别通过过氧化氢和过硫酸盐的UV光分解(即UV / H2O2和UV / PS)产生。检查了初始溶液pH,RhB浓度,氧化剂用量,Fe2 +浓度和水基质的影响。结果表明,RhB的降解在两个过程中均遵循伪一级动力学,其中UV / H2O2过程表现出比UV / PS过程更好的性能。酸性条件有利于两个系统中RhB的降解。增加氧化剂剂量或降低污染物浓度可以增强RhB的降解。当在两个系统中都添加Fe2 +时,类似光芬顿的工艺可提高性能。添加腐殖质会抑制RhB的去除效率。Cl-的添加在UV / H2O2和UV / PS系统中分别没有表现出明显的作用并促进RhB降解。NO3-的存在促进了RhB的降解,而H2PO4-和C2O42-则显示出对UV / H2O2和UV / PS过程的抑制作用。自由基清除测试表明SO4•-自由基在UV / PS系统中起主要作用。此外,比较了这两个过程中RhB降解过程中低分子量有机酸和NH4 +的演变。UV / H2O2和UV / PS系统都导致类似的NH4 +和一些开环产物(例如,甲酸,乙酸和草酸)的形成趋势,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号