当前位置:
X-MOL 学术
›
Enzyme Microb. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization and rational design for substrate specificity of a prolyl endopeptidase from Stenotrophomonas maltophilia
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.enzmictec.2020.109548 Junjie Yu 1 , Junjie Wu 1 , Dewei Xie 1 , Lei Du 1 , Ya-Jie Tang 2 , Jingli Xie 3 , Dongzhi Wei 3
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.enzmictec.2020.109548 Junjie Yu 1 , Junjie Wu 1 , Dewei Xie 1 , Lei Du 1 , Ya-Jie Tang 2 , Jingli Xie 3 , Dongzhi Wei 3
Affiliation
|
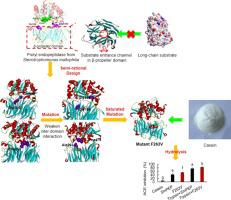
A novel prolyl endopeptidase from Stenotrophomonas maltophilia, SmPEP, was discovered and characterized. The specific activity of the recombinant SmPEP expressed by Escherichia coli BL21 (DE3), was 68.3 U/mg at pH 8.0 and 37 °C. In order to improve the substrate specificity for long-chain peptide, rational design was applied based on the structure constructed by homology modeling. Inter-domain sites within the β-propeller domain were chosen for the mutation to weaken the inter-domain interaction and form an open conformation for long-chain substrate entering into the active site. The substrate specificity on a designed long-chain substrate, PQPQLPYPQPQLP, of the mutants F263A and E184 G increased 8.77 and 5.75 times respectively versus wild-type. After the saturated mutation of the both sites, the reactive rate of mutant F263 V on 13-mer peptide was 10.2 times higher than that of the wild-type. Then the mutant F263 V was used in the hydrolysis of casein, and the ACE inhibitory activity of the hydrolysate was significantly improved compared with wild type enzyme, which verified the efficiency of the design strategy.
中文翻译:

嗜麦芽窄食单胞菌脯氨酰内肽酶底物特异性的表征和合理设计
发现并表征了一种来自嗜麦芽窄食单胞菌 SmPEP 的新型脯氨酰内肽酶。大肠杆菌 BL21 (DE3) 表达的重组 SmPEP 在 pH 8.0 和 37°C 下的比活性为 68.3 U/mg。为了提高对长链肽的底物特异性,基于同源建模构建的结构进行了合理的设计。选择β-螺旋桨结构域内的域间位点进行突变以削弱域间相互作用并形成长链底物进入活性位点的开放构象。与野生型相比,突变体 F263A 和 E184 G 对设计的长链底物 PQPQLPYPQPQLP 的底物特异性分别增加了 8.77 和 5.75 倍。两个位点饱和突变后,突变体F263 V对13-mer肽的反应率是野生型的10.2倍。然后将突变体F263 V用于酪蛋白的水解,水解产物的ACE抑制活性较野生型酶显着提高,验证了设计策略的有效性。
更新日期:2020-08-01
中文翻译:

嗜麦芽窄食单胞菌脯氨酰内肽酶底物特异性的表征和合理设计
发现并表征了一种来自嗜麦芽窄食单胞菌 SmPEP 的新型脯氨酰内肽酶。大肠杆菌 BL21 (DE3) 表达的重组 SmPEP 在 pH 8.0 和 37°C 下的比活性为 68.3 U/mg。为了提高对长链肽的底物特异性,基于同源建模构建的结构进行了合理的设计。选择β-螺旋桨结构域内的域间位点进行突变以削弱域间相互作用并形成长链底物进入活性位点的开放构象。与野生型相比,突变体 F263A 和 E184 G 对设计的长链底物 PQPQLPYPQPQLP 的底物特异性分别增加了 8.77 和 5.75 倍。两个位点饱和突变后,突变体F263 V对13-mer肽的反应率是野生型的10.2倍。然后将突变体F263 V用于酪蛋白的水解,水解产物的ACE抑制活性较野生型酶显着提高,验证了设计策略的有效性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号