Cell Reports ( IF 7.5 ) Pub Date : 2020-04-07 , DOI: 10.1016/j.celrep.2020.03.056
Katherine R Balka 1 , Cynthia Louis 2 , Tahnee L Saunders 3 , Amber M Smith 4 , Dale J Calleja 2 , Damian B D'Silva 2 , Fiona Moghaddas 2 , Maximilien Tailler 3 , Kate E Lawlor 5 , Yifan Zhan 6 , Christopher J Burns 7 , Ian P Wicks 8 , Jonathan J Miner 4 , Benjamin T Kile 9 , Seth L Masters 2 , Dominic De Nardo 1
|
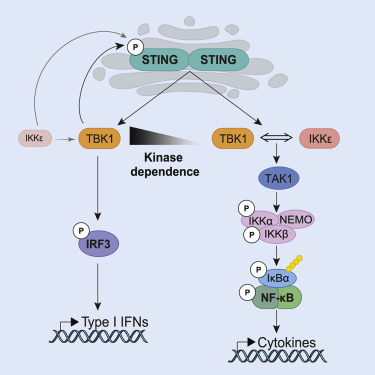
Stimulator of Interferon Genes (STING) is a critical component of host innate immune defense but can contribute to chronic autoimmune or autoinflammatory disease. Once activated, the cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) (cGAMP) synthase (cGAS)-STING pathway induces both type I interferon (IFN) expression and nuclear factor-κB (NF-κB)-mediated cytokine production. Currently, these two signaling arms are thought to be mediated by a single upstream kinase, TANK-binding kinase 1 (TBK1). Here, using genetic and pharmacological approaches, we show that TBK1 alone is dispensable for STING-induced NF-κB responses in human and mouse immune cells, as well as in vivo. We further demonstrate that TBK1 acts redundantly with IκB kinase ε (IKKε) to drive NF-κB upon STING activation. Interestingly, we show that activation of IFN regulatory factor 3 (IRF3) is highly dependent on TBK1 kinase activity, whereas NF-κB is significantly less sensitive to TBK1/IKKε kinase inhibition. Our work redefines signaling events downstream of cGAS-STING. Our findings further suggest that cGAS-STING will need to be targeted directly to effectively ameliorate the inflammation underpinning disorders associated with STING hyperactivity.
中文翻译:

TBK1 和 IKKε 在介导骨髓细胞中 STING 诱导的 NF-κB 反应中起冗余作用。
干扰素基因刺激物 (STING) 是宿主先天免疫防御的重要组成部分,但可导致慢性自身免疫性疾病或自身炎症性疾病。一旦被激活,环磷酸鸟苷 (GMP)-磷酸腺苷 (AMP) (cGAMP) 合成酶 (cGAS)-STING 通路会诱导 I 型干扰素 (IFN) 表达和核因子-κB (NF-κB) 介导的细胞因子产生。目前,这两个信号臂被认为是由单个上游激酶 TANK 结合激酶 1 (TBK1) 介导的。在这里,我们使用遗传学和药理学方法表明,单独的 TBK1 对于人和小鼠免疫细胞以及体内STING 诱导的 NF-κB 反应是可有可无的. 我们进一步证明 TBK1 与 IκB 激酶 ε (IKKε) 冗余作用以在 STING 激活时驱动 NF-κB。有趣的是,我们发现 IFN 调节因子 3 (IRF3) 的激活高度依赖于 TBK1 激酶活性,而 NF-κB 对 TBK1/IKKε 激酶抑制的敏感性明显较低。我们的工作重新定义了 cGAS-STING 下游的信号事件。我们的研究结果进一步表明,cGAS-STING 需要直接靶向以有效改善与 STING 多动症相关的炎症基础疾病。

































 京公网安备 11010802027423号
京公网安备 11010802027423号