当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine Capture Using Zr-Based Metal-Organic Frameworks (Zr-MOFs): Adsorption Performance and Mechanism.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-04-15 , DOI: 10.1021/acsami.0c02129 Peng Chen 1 , Xihong He 2 , Maobin Pang 1 , Xiuting Dong 1 , Song Zhao 1 , Wen Zhang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-04-15 , DOI: 10.1021/acsami.0c02129 Peng Chen 1 , Xihong He 2 , Maobin Pang 1 , Xiuting Dong 1 , Song Zhao 1 , Wen Zhang 1
Affiliation
|
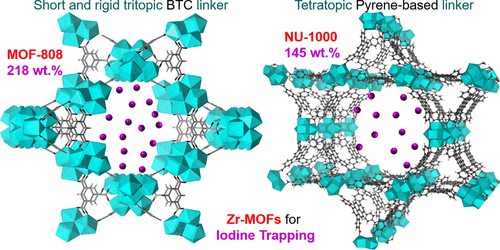
The effective capture of radioiodine, produced or released from nuclear-related activities, is of paramount importance for the sustainable development of nuclear energy. Here, a series of zirconium-based metal-organic frameworks (Zr-MOFs), with a Zr6(μ3-O)4(μ3-OH)4 cluster and various carboxylate linkers, were investigated for the capture of volatile iodine. Their adsorption kinetics and recyclability were investigated in dry and humid environments. The structural change of Zr-MOFs during iodine trapping was studied using powder X-ray diffraction and pore structure measurements. Experimental spectra (Raman and X-ray photoelectron spectroscopy) and density functional theory (DFT) calculations for the linkers and Zr clusters were performed to understand the trapping mechanism of the framework. When interacting with iodine molecules, MOF-808, NU-1000, and UiO-66, with highly connected and/or rigid linkers, have better structural stability than UiO-67 and MOF-867, which have flexible linkers with less connectivity. Particularly, MOF-808, with a rigid and tritopic benzenetricarboxylate linker, has the highest iodine adsorption capacity (2.18 g/g, 80 °C), as well as the largest pore volume after iodine elution. In contrast, UiO-67, with long linear ditopic linkers, exhibits the weakest stability and lowest adsorption capacity (0.53 g/g, 80 °C) because of its most serious collapse of pore structures. After incorporating with strong electron-donating imidazole/pyridine ligands, both the stability and adsorption capacity of MOF-808/NU-1000 decrease. DFT calculations verify that the N-heterocycle groups could enhance the affinity toward iodine by strong charge transfer. DFT calculations also suggest that the terminal -OH in MOF-808 has a strong affinity toward iodine (-54 kJ/mol I2) and water (-63 kJ/mol H2O) and a weak affinity toward NO2 (-27 kJ/mol NO2). With high adsorption capacity and excellent stability, MOF-808 shows great potential for the sustainable removal of radioiodine.
中文翻译:

使用基于Zr的金属有机框架(Zr-MOF)捕获碘:吸附性能和机理。
从核相关活动中产生或释放的放射性碘的有效捕获对于核能的可持续发展至关重要。在这里,研究了一系列具有Zr6(μ3-O)4(μ3-OH)4簇和各种羧酸盐连接基的锆基金属有机骨架(Zr-MOF),用于捕获挥发性碘。在干燥和潮湿的环境中研究了它们的吸附动力学和可回收性。利用粉末X射线衍射和孔结构测量研究了Zr-MOFs在碘捕获过程中的结构变化。对接头和Zr团簇进行了实验光谱(拉曼光谱和X射线光电子能谱)和密度泛函理论(DFT)计算,以了解框架的俘获机理。与碘分子相互作用时,MOF-808,NU-1000,具有高度连接和/或刚性接头的UiO-66和UiO-66与具有挠性接头且连通性较低的UiO-67和MOF-867具有更好的结构稳定性。尤其是,具有刚性三聚苯甲酸三苯酯连接基的MOF-808具有最高的碘吸附容量(2.18 g / g,80°C),并且在洗脱碘后具有最大的孔体积。相反,UiO-67具有长的线性对位连接基,由于其最严重的孔结构塌陷,显示出最弱的稳定性和最低的吸附容量(0.53 g / g,80°C)。与强供电子咪唑/吡啶配体结合后,MOF-808 / NU-1000的稳定性和吸附能力均下降。DFT计算证明,N-杂环基团可以通过强电荷转移增强对碘的亲和力。DFT计算还表明,MOF-808中的-OH末端对碘(-54 kJ / mol H2O)和水(-63 kJ / mol H2O)有很强的亲和力,对NO2(-27 kJ / mol NO2)有很弱的亲和力)。MOF-808具有高吸附能力和出色的稳定性,显示出可持续去除放射性碘的巨大潜力。
更新日期:2020-04-07
中文翻译:

使用基于Zr的金属有机框架(Zr-MOF)捕获碘:吸附性能和机理。
从核相关活动中产生或释放的放射性碘的有效捕获对于核能的可持续发展至关重要。在这里,研究了一系列具有Zr6(μ3-O)4(μ3-OH)4簇和各种羧酸盐连接基的锆基金属有机骨架(Zr-MOF),用于捕获挥发性碘。在干燥和潮湿的环境中研究了它们的吸附动力学和可回收性。利用粉末X射线衍射和孔结构测量研究了Zr-MOFs在碘捕获过程中的结构变化。对接头和Zr团簇进行了实验光谱(拉曼光谱和X射线光电子能谱)和密度泛函理论(DFT)计算,以了解框架的俘获机理。与碘分子相互作用时,MOF-808,NU-1000,具有高度连接和/或刚性接头的UiO-66和UiO-66与具有挠性接头且连通性较低的UiO-67和MOF-867具有更好的结构稳定性。尤其是,具有刚性三聚苯甲酸三苯酯连接基的MOF-808具有最高的碘吸附容量(2.18 g / g,80°C),并且在洗脱碘后具有最大的孔体积。相反,UiO-67具有长的线性对位连接基,由于其最严重的孔结构塌陷,显示出最弱的稳定性和最低的吸附容量(0.53 g / g,80°C)。与强供电子咪唑/吡啶配体结合后,MOF-808 / NU-1000的稳定性和吸附能力均下降。DFT计算证明,N-杂环基团可以通过强电荷转移增强对碘的亲和力。DFT计算还表明,MOF-808中的-OH末端对碘(-54 kJ / mol H2O)和水(-63 kJ / mol H2O)有很强的亲和力,对NO2(-27 kJ / mol NO2)有很弱的亲和力)。MOF-808具有高吸附能力和出色的稳定性,显示出可持续去除放射性碘的巨大潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号