当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusual Side Transformation of Spiro[2,4]hepta‐4,6‐dienes into Fulvene Derivatives During Pd‐Catalyzed Cyclopropanation with Diazomethane
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-04-06 , DOI: 10.1002/slct.202000160
Evgeny V. Shulishov 1 , Olga A. Pantyukh 1 , Leonid G. Menchikov 1 , Yury V. Tomilov 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-04-06 , DOI: 10.1002/slct.202000160
Evgeny V. Shulishov 1 , Olga A. Pantyukh 1 , Leonid G. Menchikov 1 , Yury V. Tomilov 1
Affiliation
|
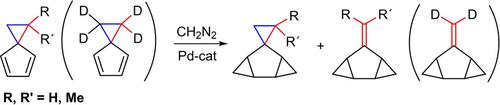
The catalytic cyclopropanation of spiro[2.4]hepta‐4,6‐diene and 1,1,2,2‐tetradeutero‐, 1‐methyl‐, and 1,1‐dimethylspiro[2.4]hepta‐4,6‐dienes on treatment with excess diazomethane in the presence of palladium catalysts, which gives products of double bond dicyclopropanation, is also accompanied by an unusual transformation of the three‐membered ring of the spiro moiety with elimination of the unsubstituted methylene group in the form of ethylene to give the corresponding 5‐methylidenetricyclo[4.1.0.02,4]heptanes (bishomofulvenes). The putative mechanism of the reactions is considered.
中文翻译:

重氮甲烷在Pd催化的环丙烷化反应中将Spiro [2,4] hepta-4,6-dienes发生不寻常的边转化为Fulven衍生物
螺[2.4]庚-4,6-二烯和1,1,2,2-四氘代,1-甲基和1,1-二甲基螺[2.4]庚-4,6-二烯在处理上的催化环丙烷化在钯催化剂存在下,用过量的重氮甲烷生成双键双环丙烷化产物,同时还伴随有螺环部分三元环的异常转变,同时消除了乙烯形式的未取代亚甲基,从而得到相应的5-亚甲基四环[4.1.0.0 2,4 ]庚烷(双酚富烯)。考虑反应的推定机理。
更新日期:2020-04-06
中文翻译:

重氮甲烷在Pd催化的环丙烷化反应中将Spiro [2,4] hepta-4,6-dienes发生不寻常的边转化为Fulven衍生物
螺[2.4]庚-4,6-二烯和1,1,2,2-四氘代,1-甲基和1,1-二甲基螺[2.4]庚-4,6-二烯在处理上的催化环丙烷化在钯催化剂存在下,用过量的重氮甲烷生成双键双环丙烷化产物,同时还伴随有螺环部分三元环的异常转变,同时消除了乙烯形式的未取代亚甲基,从而得到相应的5-亚甲基四环[4.1.0.0 2,4 ]庚烷(双酚富烯)。考虑反应的推定机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号