当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Click Synthesis of 1,2,3‐Triazoles‐Linked 1,2,4‐Triazino[5,6‐b]indole, Antibacterial Activities and Molecular Docking Studies
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-04-06 , DOI: 10.1002/slct.202000266 Ali Keivanloo 1 , Saghi Sepehri 2 , Mohammad Bakherad 1 , Mahboobe Eskandari 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-04-06 , DOI: 10.1002/slct.202000266 Ali Keivanloo 1 , Saghi Sepehri 2 , Mohammad Bakherad 1 , Mahboobe Eskandari 1
Affiliation
|
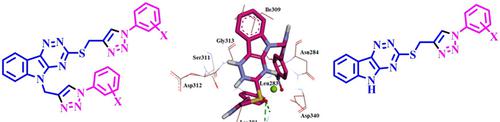
The reaction of 4,5‐dihydro‐3H‐1,2,4‐triazino[5,6‐b]indole‐3‐thion with propargyl bromide, in EtOH at 80 °C, produces mono‐propargylated 3‐(prop‐2‐one‐1‐ylthio)‐5H‐1,2,4‐triazino[5,6‐b]indole. Further reaction of this compound with propargyl bromide, in DMF in the presence of K2CO3 at room temperature, produced successfully double‐propargylated 5‐(prop‐2‐one‐1‐yl)‐3‐(prop‐2‐one‐1‐ylthio)‐5H‐1,2,4‐triazino[5,6‐b]indole. The Cu(I)‐catalyzed click reaction of these propargylated compounds with different aromatic azides at room temperature, afforded new derivatives of 1,2,3‐triazoles‐linked 1,2,4‐triazino[5,6‐b]indole with high yields. The in vitro antibacterial activities of the novel 1,2,3‐triazoles were screened. The binding modes of these compounds to three enzyme active sites were determined by a molecular docking study. Docking studies demonstrated the most potent compounds; and binding maps revealed that the activities might be attributed to the hydrophobic interactions, hydrogen bonds, cation‐π and π‐π contacts with the active sites.
中文翻译:

点击合成1,2,3-三唑连接的1,2,4-三嗪并[5,6-b]吲哚,抗菌活性和分子对接研究
4,5-二氢-3 H -1,2,4-三嗪基[ 5,6- b ]吲哚-3-硫酮与炔丙基溴在80°C的乙醇中反应,生成单炔丙基化3-(丙-2-1-1-乙硫基)-5 H -1,2,4-三嗪[5,6- b ]吲哚。在室温下在K 2 CO 3存在下,该化合物与炔丙基溴在DMF中进一步反应,成功生成了双炔丙基化的5-(prop-2-one-1-基)-3-(prop-2-one)。 ‐1‐ylthio)‐5 H ‐1,2,4‐三嗪基[5,6‐ b ]吲哚。这些炔丙基化化合物与不同的芳族叠氮化物在室温下的Cu(I)催化的点击反应,提供了1,2,3-三唑连接的1,2,4-三嗪[5,6- b]的新衍生物]吲哚具有高收率。筛选了新型1,2,3-三唑的体外抗菌活性。通过分子对接研究确定了这些化合物与三个酶活性位点的结合方式。对接研究证明了最有效的化合物。结合图表明,活性可能归因于疏水性相互作用,氢键,阳离子-π和π-π与活性位点的接触。
更新日期:2020-04-06
中文翻译:

点击合成1,2,3-三唑连接的1,2,4-三嗪并[5,6-b]吲哚,抗菌活性和分子对接研究
4,5-二氢-3 H -1,2,4-三嗪基[ 5,6- b ]吲哚-3-硫酮与炔丙基溴在80°C的乙醇中反应,生成单炔丙基化3-(丙-2-1-1-乙硫基)-5 H -1,2,4-三嗪[5,6- b ]吲哚。在室温下在K 2 CO 3存在下,该化合物与炔丙基溴在DMF中进一步反应,成功生成了双炔丙基化的5-(prop-2-one-1-基)-3-(prop-2-one)。 ‐1‐ylthio)‐5 H ‐1,2,4‐三嗪基[5,6‐ b ]吲哚。这些炔丙基化化合物与不同的芳族叠氮化物在室温下的Cu(I)催化的点击反应,提供了1,2,3-三唑连接的1,2,4-三嗪[5,6- b]的新衍生物]吲哚具有高收率。筛选了新型1,2,3-三唑的体外抗菌活性。通过分子对接研究确定了这些化合物与三个酶活性位点的结合方式。对接研究证明了最有效的化合物。结合图表明,活性可能归因于疏水性相互作用,氢键,阳离子-π和π-π与活性位点的接触。






























 京公网安备 11010802027423号
京公网安备 11010802027423号