Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-04-05 , DOI: 10.1016/j.jcat.2020.03.018
Yu-Cheng Liu , Chen-Hao Yeh , Yen-Fan Lo , Santhanamoorthi Nachimuthu , Shawn D. Lin , Jyh-Chiang Jiang
|
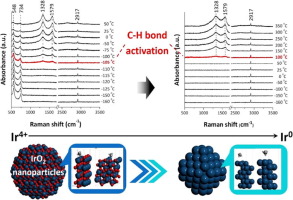
Iridium oxide (IrO2) shows a good potential for CH4 activation at mild conditions evidenced by density functional theory (DFT) and surface science studies. In this study, IrO2 NP (nanoparticles) of around 7 nm are found to activate CH4 at a temperature as low as −110 °C when Raman bands of surface species at 1328 and 1581 cm−1 are observed, accompanied by the partial reduction of IrO2. DFT modeling of methane activation on IrO2 indicates that CH3, CH2Obr, H2Obr, and HObr (Obr, bridging oxygen between two Ir) are the more abundance surface species and the calculated vibrational frequencies of these species consistently explain the observed results of in situ Raman and in situ DRIFTS. The CH4 activation temperature on Ir NP of around 5.4 nm is found nearly 200 °C higher than that over IrO2 NP, confirming the superior activity of IrO2. DFT modeling demonstrates the high CH4 reactivity over IrO2 (2 1 1) and the even higher activity of partially reduced IrO2. This evidences the important role of the oxidation state of Ir in determining the catalytic activity and it implies that tuning Ir oxidation state and/or designing Ir-oxide interface may facilitate the fabrication of value-added chemicals from methane over iridium-based catalysts.
中文翻译:

IrO 2纳米颗粒上甲烷活化的原位光谱和理论研究:Ir氧化态对CH活化的作用
氧化铱(IrO 2)在温和条件下显示出良好的CH 4活化潜力,这由密度泛函理论(DFT)和表面科学研究证明。在这项研究中,当在1328和1581 cm -1处观察到表面物种的拉曼光谱带时,发现在约-110°C的温度下,约7 nm的IrO 2 NP(纳米粒子)会激活CH 4。还原IrO 2。DFT模拟甲烷在IrO 2上的活化表明CH 3,CH 2 O br,H 2 O br和HO br(O br,在两个Ir之间桥接氧是更丰富的表面物质,并且这些物质的计算振动频率一致地解释了原位拉曼和原位DRIFTS的观察结果。的CH 4上的Ir NP活化温度纳米周围5.4的是发现了近200℃高于以上的IrO 2 NP,确认的IrO的优良活性2。DFT建模表明,与IrO 2(2 1 1)相比,CH 4具有较高的反应活性,部分还原的IrO 2具有更高的活性。这证明了Ir的氧化态在确定催化活性中的重要作用,这意味着调整Ir的氧化态和/或设计Ir-氧化物的界面可以促进甲烷在铱基催化剂上制备增值化学品。



































 京公网安备 11010802027423号
京公网安备 11010802027423号