Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-04-06 , DOI: 10.1016/j.tetlet.2020.151917 Jie Wu , Wei Liu , Li Liang , Ya Gan , Shuang Xia , Xiaojun Gou , Xiaohua Sun
|
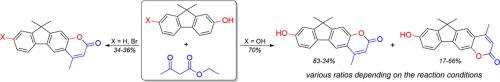
The Pechmann condensation of phenolic fluorenes with ethyl acetoacetate afforded indene-fused 4-methylcoumarins. In addition to the expected indeno[1,2-g]coumarin, a skeletal rearranged compound was obtained as a concomitant product when 9,9-dimethyl-9H-fluorene-2,7-diol was utilized as the substrate. X-ray crystallography was used to identify the structures of the isomers. Photochemical and photophysical studies indicated that compared with the non-rearranged products, the skeletal rearranged isomers have red shifted absorption maxima and much higher fluorescence quantum yields. A mechanism involving two competitive pathways for the simultaneous production of isomers was proposed.
中文翻译:

茚满融合的4-甲基香豆素的简便合成与表征以及通过Pechmann缩合进行的意外骨架重排
酚芴与乙酰乙酸乙酯的Pechmann缩合反应得到茚-稠合的4-甲基香豆素。当将9,9-二甲基-9 H-芴-2,7-二醇用作底物时,除了预期的茚并[1,2- g ]香豆素以外,还获得了骨架重排化合物。X射线晶体学用于鉴定异构体的结构。光化学和光物理研究表明,与非重排产物相比,骨架重排异构体具有最大吸收红移和更高的荧光量子产率。提出了同时涉及异构体生产的两个竞争途径的机制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号