当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Addition of Aryl Halides to a Triphosphine Ni(0) Center to Form Pentacoordinate Ni(II) Aryl Species.
Organometallics ( IF 2.5 ) Pub Date : 2020-04-03 , DOI: 10.1021/acs.organomet.0c00060 Pablo M Pérez García 1 , Andrea Darù 2 , Arthur R Scheerder 1 , Martin Lutz 3 , Jeremy N Harvey 2 , Marc-Etienne Moret 1
Organometallics ( IF 2.5 ) Pub Date : 2020-04-03 , DOI: 10.1021/acs.organomet.0c00060 Pablo M Pérez García 1 , Andrea Darù 2 , Arthur R Scheerder 1 , Martin Lutz 3 , Jeremy N Harvey 2 , Marc-Etienne Moret 1
Affiliation
|
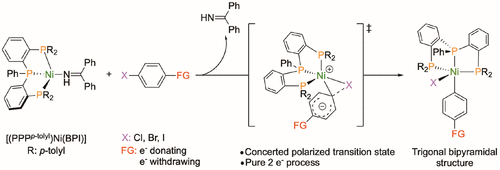
Oxidative addition of aryl halides to Ni(0) is a ubiquitous elementary step in cross-coupling and related reactions, usually producing a square-planar Ni(II)-aryl intermediate. Here we show that a triphosphine ligand supports oxidative addition at a tris-ligated Ni(0) center to cleanly form stable five-coordinate Ni(II)-aryl compounds. Kinetic and computational studies support a concerted, two-electron mechanism rather than radical halogen abstraction. These results support the idea that oxidative addition to triphosphine Ni(0) species may be more generally involved in Ni/phosphine catalytic systems.
中文翻译:

芳基卤化物向三膦Ni(0)中心的氧化加成反应生成五配位Ni(II)芳基物种。
芳基卤化物向Ni(0)的氧化加成是交叉偶联和相关反应中普遍存在的基本步骤,通常会产生方形的Ni(II)-芳基中间体。在这里,我们表明三膦配体在三连接的Ni(0)中心支持氧化加成,以干净地形成稳定的五配位Ni(II)-芳基化合物。动力学和计算研究支持协同的双电子机理,而不是自由基卤素的提取。这些结果支持这样的想法,即氧化成三膦Ni(0)物质可能更普遍地涉及Ni /膦催化系统。
更新日期:2020-04-03
中文翻译:

芳基卤化物向三膦Ni(0)中心的氧化加成反应生成五配位Ni(II)芳基物种。
芳基卤化物向Ni(0)的氧化加成是交叉偶联和相关反应中普遍存在的基本步骤,通常会产生方形的Ni(II)-芳基中间体。在这里,我们表明三膦配体在三连接的Ni(0)中心支持氧化加成,以干净地形成稳定的五配位Ni(II)-芳基化合物。动力学和计算研究支持协同的双电子机理,而不是自由基卤素的提取。这些结果支持这样的想法,即氧化成三膦Ni(0)物质可能更普遍地涉及Ni /膦催化系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号