当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Asp190 in the Phosphorylation of the Antibiotic Kanamycin Catalyzed by the Aminoglycoside Phosphotransferase Enzyme: A Combined QM:QM and MD Study.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-04-17 , DOI: 10.1021/acs.jpcb.0c01604 Abd Al-Aziz A Abu-Saleh 1 , Sweta Sharma 2 , Arpita Yadav 2 , Raymond A Poirier 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-04-17 , DOI: 10.1021/acs.jpcb.0c01604 Abd Al-Aziz A Abu-Saleh 1 , Sweta Sharma 2 , Arpita Yadav 2 , Raymond A Poirier 1
Affiliation
|
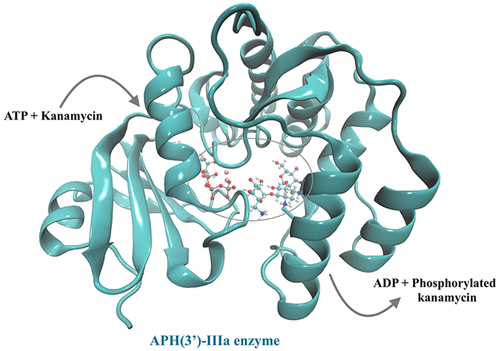
The aminoglycoside phosphotransferase (APH(3')-IIIa) kinases form a clinically central group of antibiotic-resistant enzymes. Computationally, we have studied the catalytic mechanism of the APH(3')-IIIa enzyme at the atomic-level. The proposed reaction mechanism involves protonation of Asp190 by the kanamycin 3'-hydroxyl group mediated through an explicit neighboring water molecule, which leads to a simultaneous nucleophilic attack on the γ-phosphate of the ATP by the deprotonated kanamycin 3'-hydroxyl group. The second step is a proton abstraction from the protonated Asp190 to the phosphate group of the phosphorylated kanamycin mediated by an explicit water molecule. The calculated Gibbs energy of activation (ΔG⧧) of the rate-determining step for the phosphorylation reaction is 77 kJ mol-1 at the M06-2X/6-311++G(2df,p)//ONIOM(M06-2X/6-31+G(d):HF/6-31G(d)) level of theory. This study has provided a new understanding of the APH(3')-IIIa catalytic mechanism that agrees with the available experimental data (ΔG⧧ = 75 ± 4 kJ mol-1) and could provide a starting point for the rational design of mechanism-based inhibitors of aminoglycoside modifying enzyme to circumvent antibiotic resistance.
中文翻译:

Asp190在氨基糖苷磷酸转移酶催化的抗生素卡那霉素磷酸化中的作用:结合QM:QM和MD研究。
氨基糖苷磷酸转移酶(APH(3')-IIIa)激酶形成了抗生素耐药性酶的临床中心组。通过计算,我们已经在原子水平上研究了APH(3')-IIIa酶的催化机理。拟议的反应机制涉及通过明显的邻近水分子介导的卡那霉素3'-羟基使Asp190质子化,从而导致去质子化的卡那霉素3'-羟基同时对ATP的γ-磷酸产生亲核攻击。第二步是从质子化的Asp190到质子化水分子介导的磷酸化卡那霉素的磷酸基团的质子提取。在M06-2X / 6-311 ++ G(2df)上,磷酸化反应速率确定步骤的计算吉布斯活化能(ΔG⧧)为77 kJ mol-1。p)// ONIOM(M06-2X / 6-31 + G(d):HF / 6-31G(d))的理论水平。这项研究为APH(3')-IIIa催化机理提供了新的认识,与现有的实验数据(ΔG⧧= 75±4 kJ mol-1)相符,并且可以为合理设计机理提供一个起点-为基础的氨基糖苷修饰酶抑制剂来规避抗生素耐药性。
更新日期:2020-04-24
中文翻译:

Asp190在氨基糖苷磷酸转移酶催化的抗生素卡那霉素磷酸化中的作用:结合QM:QM和MD研究。
氨基糖苷磷酸转移酶(APH(3')-IIIa)激酶形成了抗生素耐药性酶的临床中心组。通过计算,我们已经在原子水平上研究了APH(3')-IIIa酶的催化机理。拟议的反应机制涉及通过明显的邻近水分子介导的卡那霉素3'-羟基使Asp190质子化,从而导致去质子化的卡那霉素3'-羟基同时对ATP的γ-磷酸产生亲核攻击。第二步是从质子化的Asp190到质子化水分子介导的磷酸化卡那霉素的磷酸基团的质子提取。在M06-2X / 6-311 ++ G(2df)上,磷酸化反应速率确定步骤的计算吉布斯活化能(ΔG⧧)为77 kJ mol-1。p)// ONIOM(M06-2X / 6-31 + G(d):HF / 6-31G(d))的理论水平。这项研究为APH(3')-IIIa催化机理提供了新的认识,与现有的实验数据(ΔG⧧= 75±4 kJ mol-1)相符,并且可以为合理设计机理提供一个起点-为基础的氨基糖苷修饰酶抑制剂来规避抗生素耐药性。































 京公网安备 11010802027423号
京公网安备 11010802027423号