当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Properties and Crystal Structure of Li+/H+ Cation-Exchanged LiNiO2
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-04-03 00:00:00 , DOI: 10.1021/acsaem.0c00602 Takahiro Toma 1, 2 , Ryo Maezono 2, 3 , Kenta Hongo 3, 4, 5, 6
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-04-03 00:00:00 , DOI: 10.1021/acsaem.0c00602 Takahiro Toma 1, 2 , Ryo Maezono 2, 3 , Kenta Hongo 3, 4, 5, 6
Affiliation
|
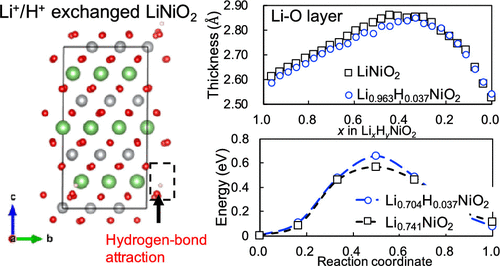
LiNiO2 has high energy density but readily reacts with moisture in the atmosphere and deteriorates. We performed qualitative and quantitative evaluations of the degraded phase of LiNiO2 and the influence of the structural change on the electrochemical properties of the phase. The formation of the Li1–xHxNiO2 phase with cation exchange between Li+ and H+ was confirmed by thermogravimetric analysis and Karl Fischer titration measurement. As the H concentration in Li1–xHxNiO2 increased, the rate capability deteriorated, especially in the low-temperature range and under low state of charge. Experimental and density functional theory (DFT) calculation results suggested that this outcome was attributed to an increased activation energy of Li+ diffusion because of cation exchange. Rietveld analysis of X-ray diffraction and DFT calculation confirmed that the c lattice parameter and Li–O layer decreased because of the Li+/H+ cation exchange. These results indicate that LiNiO2 reacting with moisture in the atmosphere has a narrowed Li–O layer, which is the Li diffusion path, and the rate characteristics are degraded.
中文翻译:

Li + / H +阳离子交换的LiNiO 2的电化学性质和晶体结构
LiNiO 2具有高能量密度,但是容易与大气中的水分反应并劣化。我们对LiNiO 2的降解相进行了定性和定量评估,以及结构变化对该相的电化学性质的影响。通过热重分析和卡尔·费休滴定法确定了在Li +和H +之间发生阳离子交换的Li 1– x H x NiO 2相的形成。Li 1– x H x NiO 2中的H浓度增加,速率能力恶化,特别是在低温范围和低电荷状态下。实验和密度泛函理论(DFT)的计算结果表明,该结果归因于由于阳离子交换,Li +扩散的活化能增加。Rietveld的X射线衍射分析和DFT计算证实,由于Li + / H +阳离子交换,c晶格参数和Li–O层减少。这些结果表明,与大气中的水分反应的LiNiO 2具有狭窄的Li–O层,这是Li的扩散路径,并且速率特性下降。
更新日期:2020-04-03
中文翻译:

Li + / H +阳离子交换的LiNiO 2的电化学性质和晶体结构
LiNiO 2具有高能量密度,但是容易与大气中的水分反应并劣化。我们对LiNiO 2的降解相进行了定性和定量评估,以及结构变化对该相的电化学性质的影响。通过热重分析和卡尔·费休滴定法确定了在Li +和H +之间发生阳离子交换的Li 1– x H x NiO 2相的形成。Li 1– x H x NiO 2中的H浓度增加,速率能力恶化,特别是在低温范围和低电荷状态下。实验和密度泛函理论(DFT)的计算结果表明,该结果归因于由于阳离子交换,Li +扩散的活化能增加。Rietveld的X射线衍射分析和DFT计算证实,由于Li + / H +阳离子交换,c晶格参数和Li–O层减少。这些结果表明,与大气中的水分反应的LiNiO 2具有狭窄的Li–O层,这是Li的扩散路径,并且速率特性下降。






























 京公网安备 11010802027423号
京公网安备 11010802027423号