Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.molliq.2020.113042 Dhaybia Douche , Hicham Elmsellem , El Hassane Anouar , Lei Guo , Baraa Hafez , Burak Tüzün , Ahmed El Louzi , Khalid Bougrin , Khalid Karrouchi , Banacer Himmi
|
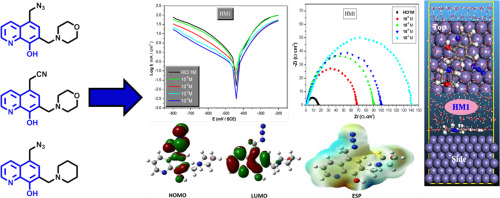
The anti-corrosion potency of three synthesized 8-hydroxyquinoline derivatives, namely 5-(azidomethyl)-7-(morpholinomethyl)quinolin-8-ol (HM1), 2-(8-hydroxy-7-(morpholinomethyl)quinolin-5-yl)acetonitrile (HM2), 5-(azidomethyl)-7-(piperidin-1-ylmethyl)quinolin-8-ol (HM3) in hydrochloric acid for mild steel was investigated using weight loss and electrochemical techniques. Potentiodynamic polarization (PDP) data reveal that all three compounds were cathodic inhibitors, with HM3 presentation significant mixed-type effect at high inhibitor concentrations (10−3 M). Electrochemical impedance spectroscopy (EIS) data reveal better adsorption of compounds species on MS surface at increased inhibitor concentrations with HM1, HM2 and HM3 reaching a maximum efficiency of 90, 89 and 88%. The three compounds HM1, HM2 and HM3 were inclined towards the Langmuir adsorption-isotherm by spontaneous chemical-physical adsorptions of inhibitors on the mild steel surface. The correlation between the electronic properties and inhibition efficacies of the tilted inhibitors was determined by using simple linear regression technique. Electronic properties were calculated for neutral and protonated forms in a polarizable continuum model using the DFT method at the B3LYP/6–311 + G (d, p) level of theory. The active adsorbed sites of HM1-HM3 on the metal surface were determined by analyzing their corresponding electrostatic surface potentials (ESP). Furthermore, molecular dynamics simulations have been performed to illustrate the most conceivable adsorption configuration between the inhibitors and metal surface.
中文翻译:

8-羟基喹啉衍生物在酸性介质中对低碳钢的耐腐蚀性能:重量,电化学,DFT和分子动力学模拟研究
三种合成的8-羟基喹啉衍生物(5-(叠氮基甲基)-7-(吗啉代甲基)喹啉-8-醇(HM1),2-(8-羟基-7-(吗啉代甲基)喹啉-5-)的抗腐蚀能力利用失重法和电化学技术研究了在低碳钢中使用的丁基)乙腈(HM2),5-(叠氮基甲基)-7-(哌啶-1-基甲基)喹啉-8-醇(HM3)。电位动力学极化(PDP)数据表明,所有三种化合物均为阴极抑制剂,HM3在高抑制剂浓度下表现出明显的混合型效应(10 -3 M)。电化学阻抗谱(EIS)数据显示,在抑制剂浓度增加的情况下,HM1,HM2和HM3的化合物种类在MS表面的吸附效果更好,最大效率达到90%,89%和88%。三种化合物HM1,HM2和HM3由于缓蚀剂在低碳钢表面上的自发化学物理吸附,它们倾向于Langmuir吸附等温线。通过使用简单的线性回归技术确定了倾斜抑制剂的电子性质与抑制效率之间的相关性。在B3LYP / 6–311 + G(d,p)的理论水平下,使用DFT方法在可极化的连续体模型中计算了中性和质子化形式的电子性质。通过分析其相应的静电表面电势(ESP),确定金属表面上HM1 - HM3的活性吸附位。此外,已经进行了分子动力学模拟以说明抑制剂和金属表面之间最可能的吸附构型。






























 京公网安备 11010802027423号
京公网安备 11010802027423号