当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antimalarial Eudesmane Sesquiterpenoids from Dobinea delavayi.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.jnatprod.9b00761 Yi Shen , Shu-Jun Cui , Hao Chen , Lei Shen , Min Wang , Xiang Dong , Chao-Jiang Xiao , Bei Jiang
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.jnatprod.9b00761 Yi Shen , Shu-Jun Cui , Hao Chen , Lei Shen , Min Wang , Xiang Dong , Chao-Jiang Xiao , Bei Jiang
|
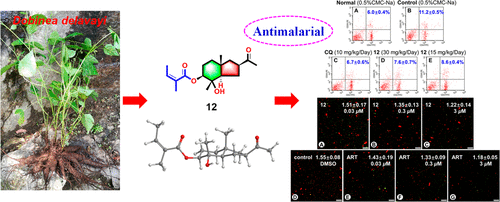
Eleven new angeloylated eudesmane sesquiterpenoids, dobinins D-N (2, 3, 5, 6, 8, 9, and 11-15), and four known compounds (1, 4, 7, and 10) were isolated from the roots of Dobinea delavayi. A new oxidation product (8a) was also obtained from dobinin H (8). Their structures were elucidated by spectroscopic data and single-crystal X-ray diffraction analyses. Dobinins K-N (12-15) are the first examples of rearrangement noreudesmane analogue sesquiterpenoids with a unique 6/5-fused carbon skeleton. A putative biosynthetic pathway of compounds 12-15 is proposed. Compound 12 exhibited significant antimalarial activity superior to artemisinin with the inhibition ratio of 59.1%, and compounds 3, 5, and 15 exhibited moderate antimalarial activities against Plasmodium yoelii BY265RFP with inhibition ratios ranging from 14.5% to 18.5% at a dose of 30 mg/kg/day. In addition, the apoptosis of P. yoelii BY265RFP by the depolarization of mitochondrial membrane potential with striking ROS production, after parasitized erythrocyte lysis mediated by cytokines IL-12 and IFN-γ, may be a possible mechanism of antimalarial action of compound 12 against P. yoelii BY265RFP.
中文翻译:

来自杜比娜(Dobinea delavayi)的抗疟Eudesmane倍半萜类化合物。
从Dobinea delavayi的根中分离出了十一种新的天使化的杜鹃花倍半萜类化合物,多巴宁DN(2、3、5、6、8、9和11-15)和四种已知化合物(1、4、7和10)。还从多比宁H(8)中获得了新的氧化产物(8a)。通过光谱数据和单晶X射线衍射分析阐明了它们的结构。Dobinins KN(12-15)是具有独特的6/5融合碳骨架的重排noreudesmane类似倍半萜的第一个实例。提出了化合物12-15的推测的生物合成途径。化合物12表现出明显优于青蒿素的显着抗疟活性,抑制率为59.1%,化合物3、5和15表现出对约氏疟原虫BY265RFP的中度抗疟活性,抑制率范围为14.5%至18。剂量为5%(30毫克/千克/天)。另外,在由细胞因子IL-12和IFN-γ介导的红细胞裂解被寄生化后,线粒体膜电位去极化并产生强烈的ROS导致约氏疟原虫BY265RFP的凋亡可能是化合物12对P的抗疟作用的可能机制。 yoelii BY265RFP。
更新日期:2020-04-01
中文翻译:

来自杜比娜(Dobinea delavayi)的抗疟Eudesmane倍半萜类化合物。
从Dobinea delavayi的根中分离出了十一种新的天使化的杜鹃花倍半萜类化合物,多巴宁DN(2、3、5、6、8、9和11-15)和四种已知化合物(1、4、7和10)。还从多比宁H(8)中获得了新的氧化产物(8a)。通过光谱数据和单晶X射线衍射分析阐明了它们的结构。Dobinins KN(12-15)是具有独特的6/5融合碳骨架的重排noreudesmane类似倍半萜的第一个实例。提出了化合物12-15的推测的生物合成途径。化合物12表现出明显优于青蒿素的显着抗疟活性,抑制率为59.1%,化合物3、5和15表现出对约氏疟原虫BY265RFP的中度抗疟活性,抑制率范围为14.5%至18。剂量为5%(30毫克/千克/天)。另外,在由细胞因子IL-12和IFN-γ介导的红细胞裂解被寄生化后,线粒体膜电位去极化并产生强烈的ROS导致约氏疟原虫BY265RFP的凋亡可能是化合物12对P的抗疟作用的可能机制。 yoelii BY265RFP。





























 京公网安备 11010802027423号
京公网安备 11010802027423号