JAMA ( IF 63.1 ) Pub Date : 2020-05-19 , DOI: 10.1001/jama.2020.5388 Joseph Hadaya 1 , Max Schumm 1 , Edward H Livingston 1, 2
|
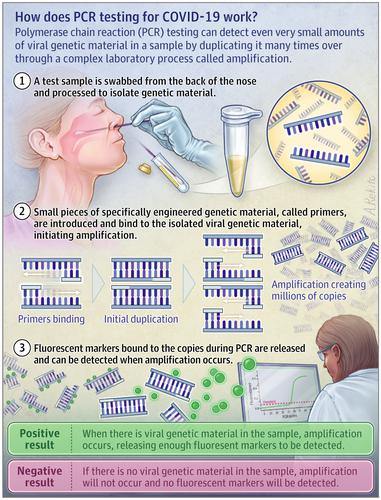
Coronavirus disease 2019 (COVID-19) infection can be diagnosed using a test called polymerase chain reaction (PCR).
Samples are taken from places likely to have the virus that causes COVID-19, like the back of the nose or mouth or deep inside the lungs. After a sample is collected, RNA, which is part of the virus particle, is extracted and converted to complementary DNA for testing. The PCR test involves binding sequences on the DNA that only are found in the virus and repeatedly copying everything in between. This process is repeated many times, with doubling of the target region with each cycle. A fluorescent signal is created when amplification occurs, and once the signal reaches a threshold, the test result is considered positive. If no viral sequence is present, amplification will not occur, resulting in a negative result.
Guidelines for testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, continue to evolve as knowledge of COVID-19 improves and availability of testing increases. Currently, testing in the US is only performed for individuals when a positive result will change treatment. Testing is also prioritized for people who have a high risk for bad outcomes from COVID-19 infection, such as elderly or immunosuppressed patients, and those with high risk of exposure and transmission of the disease to other people, such as health care workers. Recommendations for testing are regularly updated by the Centers for Disease Control and Prevention (CDC). If you have questions about testing, contact your local public health department.
中文翻译:

测试个人冠状病毒疾病2019(COVID-19)。
冠状病毒病2019(COVID-19)感染可以使用称为聚合酶链反应(PCR)的测试进行诊断。
样本是从可能带有引起COVID-19的病毒的地方采集的,例如鼻子的背面或嘴巴或肺部深处。收集样品后,提取作为病毒颗粒一部分的RNA,并将其转化为互补DNA以进行测试。PCR测试涉及仅在病毒中发现的DNA结合序列,然后重复复制两者之间的所有内容。此过程重复很多次,每个周期目标区域加倍。放大时会产生荧光信号,一旦信号达到阈值,测试结果即为阳性。如果不存在病毒序列,则不会发生扩增,从而导致阴性结果。
严重急性呼吸综合征冠状病毒2(SARS-CoV-2)(负责COVID-19的病毒)的测试指南随着对COVID-19的了解的提高和测试可用性的提高而不断发展。当前,只有在阳性结果会改变治疗的情况下,才在美国进行测试。对于那些因COVID-19感染而导致不良后果的高风险的人(例如老年人或免疫抑制的患者),以及那些容易将疾病传染给其他人的风险(例如医护人员)的人,也要优先进行测试。疾病控制与预防中心(CDC)会定期更新测试建议。如果您对测试有疑问,请联系当地的公共卫生部门。































 京公网安备 11010802027423号
京公网安备 11010802027423号