当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorine Substitution in Magnesium Hydride as a Tool for Thermodynamic Control
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-04-16 , DOI: 10.1021/acs.jpcc.9b11211
Terry D. Humphries 1 , Jack Yang 2, 3 , Richard A. Mole 2 , Mark Paskevicius 1 , Julianne E. Bird 1 , Matthew R. Rowles 1, 4 , Mariana S. Tortoza 1 , M. Veronica Sofianos 1 , Dehong Yu 2 , Craig. E. Buckley 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-04-16 , DOI: 10.1021/acs.jpcc.9b11211
Terry D. Humphries 1 , Jack Yang 2, 3 , Richard A. Mole 2 , Mark Paskevicius 1 , Julianne E. Bird 1 , Matthew R. Rowles 1, 4 , Mariana S. Tortoza 1 , M. Veronica Sofianos 1 , Dehong Yu 2 , Craig. E. Buckley 1
Affiliation
|
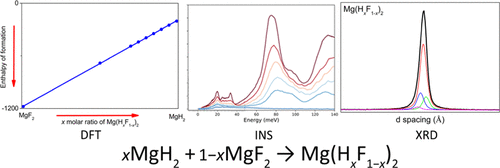
Metal hydrides continue to vie for attention as materials in multiple technological applications including hydrogen storage media, thermal energy storage (TES) materials, and hydrogen compressors. These applications depend on the temperature at which the materials desorb and reabsorb hydrogen. Magnesium hydride is ideal as a TES material, although its practical operating temperature is capped at ∼450 °C because of material degradation and high operating pressure. Fluorine substitution for hydrogen in magnesium hydride has previously been shown to increase the operating temperature of the metal hydride while limiting degradation, although full characterization is required before technological application can be ensured. The present study characterizes Mg(HxF1–x)2 solid solutions (x = 1, 0.95, 0.70, 0.85, 0.50, and 0) by inelastic neutron spectroscopy, powder X-ray diffraction, and thermal conductivity measurements, with the results being verified by density functional theory. For each experiment, a clear trend is observed throughout a series of solid solutions, showing the possibility of tuning the properties of MgH2. As F– substitution increases, the average Mg–H(F) bond distance elongates along the axial positions of the Mg–H(F) octahedra. Overall, this leads to an increase in Mg–H bond strength and thermal stability, improving the viability of Mg–H–F as potential TES materials.
中文翻译:

氟化镁中的氟取代作为热力学控制的工具
金属氢化物作为多种技术应用中的材料继续引起关注,包括氢存储介质,热能存储(TES)材料和氢压缩机。这些应用取决于材料解吸和再吸收氢的温度。氢化镁是理想的TES材料,尽管由于材料降解和较高的工作压力,其实际工作温度限制在〜450°C。以前已经证明,用氟化氢取代氢化镁中的氢可以提高金属氢化物的工作温度,同时限制降解,尽管在确保技术应用之前需要充分表征。本研究表征了Mg(H x F 1– x)非弹性中子光谱法,粉末X射线衍射法和热导率法测定2种固溶体(x = 1、0.95、0.70、0.85、0.50和0),并通过密度泛函理论进行了验证。对于每个实验,在一系列固溶体中都观察到了明显的趋势,表明有可能调节MgH 2的性质。随着F –取代的增加,平均Mg–H(F)键距沿Mg–H(F)八面体的轴向位置延长。总体而言,这导致Mg-H键强度和热稳定性的提高,提高了Mg-H-F作为潜在TES材料的可行性。
更新日期:2020-04-24
中文翻译:

氟化镁中的氟取代作为热力学控制的工具
金属氢化物作为多种技术应用中的材料继续引起关注,包括氢存储介质,热能存储(TES)材料和氢压缩机。这些应用取决于材料解吸和再吸收氢的温度。氢化镁是理想的TES材料,尽管由于材料降解和较高的工作压力,其实际工作温度限制在〜450°C。以前已经证明,用氟化氢取代氢化镁中的氢可以提高金属氢化物的工作温度,同时限制降解,尽管在确保技术应用之前需要充分表征。本研究表征了Mg(H x F 1– x)非弹性中子光谱法,粉末X射线衍射法和热导率法测定2种固溶体(x = 1、0.95、0.70、0.85、0.50和0),并通过密度泛函理论进行了验证。对于每个实验,在一系列固溶体中都观察到了明显的趋势,表明有可能调节MgH 2的性质。随着F –取代的增加,平均Mg–H(F)键距沿Mg–H(F)八面体的轴向位置延长。总体而言,这导致Mg-H键强度和热稳定性的提高,提高了Mg-H-F作为潜在TES材料的可行性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号