当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new class of ionically conducting fluorinated ether electrolytes with high electrochemical stability
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-04-01 , DOI: 10.1021/jacs.9b11056
Chibueze V Amanchukwu , Zhiao Yu , Xian Kong , Jian Qin , Yi Cui 1 , Zhenan Bao
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-04-01 , DOI: 10.1021/jacs.9b11056
Chibueze V Amanchukwu , Zhiao Yu , Xian Kong , Jian Qin , Yi Cui 1 , Zhenan Bao
Affiliation
|
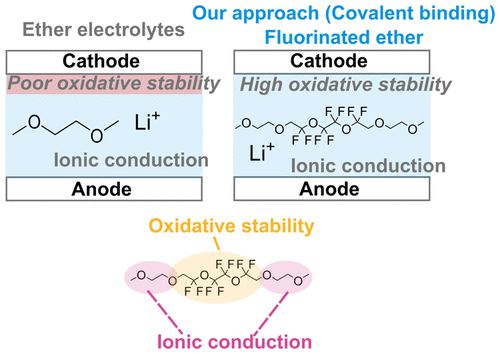
Increasing battery energy density is greatly desired for applications such as portable electronics and transportation. However, many next generation batteries are limited by electrolyte selection because high ionic conductivity and poor electrochemical stability are typically observed in most electrolytes. For example, ether-based electrolytes have high ionic conductivity, but are oxidatively unstable above 4 V, which prevents the use of high voltage cathodes that promise higher energy densities. In contrast, hydrofluoroethers (HFEs) have high oxidative stability, but do not dissolve lithium salt. In this work, we synthesize a new class of fluorinated ether electrolytes that combine the oxidative stability of HFEs with the ionic conductivity of ethers in a single compound. We show that conductivities of up to 2.7×10⁻4 S/cm (at 30°C) can be obtained with oxidative stability up to 5.6 V. The compounds also show higher lithium transference numbers compared to typical ethers. Furthermore, we use nuclear magnetic resonance (NMR) and molecular dynamics (MD) to study their ionic transport behavior and ion solvation environment, respectively. Finally, we demonstrate that these new class of electrolytes can be used with a Ni-rich layered cathode (NMC 811) to obtain over 100 cycles at a C/5 rate. The design of new molecules with high ionic conductivity and high electrochemical stability is a novel approach for the rational design of next generation batteries.
中文翻译:

一类具有高电化学稳定性的新型离子导电氟化醚电解质
对于便携式电子产品和运输等应用,非常需要增加电池能量密度。然而,许多下一代电池受到电解质选择的限制,因为在大多数电解质中通常会观察到高离子电导率和较差的电化学稳定性。例如,基于醚的电解质具有高离子电导率,但在 4 V 以上时氧化不稳定,这阻碍了承诺更高能量密度的高压阴极的使用。相比之下,氢氟醚 (HFE) 具有高氧化稳定性,但不溶解锂盐。在这项工作中,我们合成了一类新的氟化醚电解质,它将 HFE 的氧化稳定性与醚的离子电导率结合在一个化合物中。我们证明了高达 2 的电导率。可以获得 7×10⁻4 S/cm(在 30°C 下),氧化稳定性高达 5.6 V。与典型的醚相比,这些化合物还显示出更高的锂转移数。此外,我们分别使用核磁共振 (NMR) 和分子动力学 (MD) 来研究它们的离子传输行为和离子溶剂化环境。最后,我们证明了这些新型电解质可与富镍层状阴极 (NMC 811) 一起使用,以 C/5 倍率获得超过 100 次循环。设计具有高离子电导率和高电化学稳定性的新分子是合理设计下一代电池的新方法。我们分别使用核磁共振 (NMR) 和分子动力学 (MD) 来研究它们的离子传输行为和离子溶剂化环境。最后,我们证明了这些新型电解质可与富镍层状阴极 (NMC 811) 一起使用,以 C/5 倍率获得超过 100 次循环。设计具有高离子电导率和高电化学稳定性的新分子是合理设计下一代电池的新方法。我们分别使用核磁共振 (NMR) 和分子动力学 (MD) 来研究它们的离子传输行为和离子溶剂化环境。最后,我们证明了这些新型电解质可与富镍层状阴极 (NMC 811) 一起使用,以 C/5 倍率获得超过 100 次循环。设计具有高离子电导率和高电化学稳定性的新分子是合理设计下一代电池的新方法。
更新日期:2020-04-01
中文翻译:

一类具有高电化学稳定性的新型离子导电氟化醚电解质
对于便携式电子产品和运输等应用,非常需要增加电池能量密度。然而,许多下一代电池受到电解质选择的限制,因为在大多数电解质中通常会观察到高离子电导率和较差的电化学稳定性。例如,基于醚的电解质具有高离子电导率,但在 4 V 以上时氧化不稳定,这阻碍了承诺更高能量密度的高压阴极的使用。相比之下,氢氟醚 (HFE) 具有高氧化稳定性,但不溶解锂盐。在这项工作中,我们合成了一类新的氟化醚电解质,它将 HFE 的氧化稳定性与醚的离子电导率结合在一个化合物中。我们证明了高达 2 的电导率。可以获得 7×10⁻4 S/cm(在 30°C 下),氧化稳定性高达 5.6 V。与典型的醚相比,这些化合物还显示出更高的锂转移数。此外,我们分别使用核磁共振 (NMR) 和分子动力学 (MD) 来研究它们的离子传输行为和离子溶剂化环境。最后,我们证明了这些新型电解质可与富镍层状阴极 (NMC 811) 一起使用,以 C/5 倍率获得超过 100 次循环。设计具有高离子电导率和高电化学稳定性的新分子是合理设计下一代电池的新方法。我们分别使用核磁共振 (NMR) 和分子动力学 (MD) 来研究它们的离子传输行为和离子溶剂化环境。最后,我们证明了这些新型电解质可与富镍层状阴极 (NMC 811) 一起使用,以 C/5 倍率获得超过 100 次循环。设计具有高离子电导率和高电化学稳定性的新分子是合理设计下一代电池的新方法。我们分别使用核磁共振 (NMR) 和分子动力学 (MD) 来研究它们的离子传输行为和离子溶剂化环境。最后,我们证明了这些新型电解质可与富镍层状阴极 (NMC 811) 一起使用,以 C/5 倍率获得超过 100 次循环。设计具有高离子电导率和高电化学稳定性的新分子是合理设计下一代电池的新方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号