当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Restructuring a Deep Eutectic Solvent by Water: The Nanostructure of Hydrated Choline Chloride/Urea.
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2020-04-10 , DOI: 10.1021/acs.jctc.0c00120 Liel Sapir 1 , Daniel Harries 2
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2020-04-10 , DOI: 10.1021/acs.jctc.0c00120 Liel Sapir 1 , Daniel Harries 2
Affiliation
|
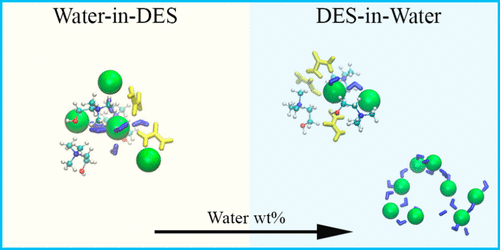
Deep eutectic mixtures are a promising sustainable and diverse class of tunable solvents that hold great promise for various green chemical and technological processes. Many deep eutectic solvents (DES) are hygroscopic and find use in applications with varying extents of hydration, hence urging a profound understanding of changes in the nanostructure of DES with water content. Here, we report on molecular dynamics simulations of the quintessential choline chloride-urea mixture, using a newly parametrized force field with scaled charges to account for physical properties of hydrated DES mixtures. These simulations indicate that water changes the nanostructure of solution even at very low hydration. We present a novel approach that uses convex constrained analysis to dissect radial distribution functions into base components representing different modes of local association. Specifically, DES mixtures can be deconvoluted locally into two dominant competing nanostructures, whose relative prevalence (but not their salient structural features) change with added water over a wide concentration range, from dry up to ∼30 wt % hydration. Water is found to be associated strongly with several DES components but remarkably also forms linear bead-on-string clusters with chloride. At high water content (beyond ∼50 wt % of water), the solution changes into an aqueous electrolyte-like mixture. Finally, the structural evolution of the solution at the nanoscale with extent of hydration is echoed in the DES macroscopic material properties. These changes to structure, in turn, should prove important in the way DES acts as a solvent and to its interactions with additive components.
中文翻译:

用水重构深的共晶溶剂:水合氯化胆碱/尿素的纳米结构。
深共晶混合物是有前途的,可持续的,多样化的可调溶剂类别,对各种绿色化学和工艺过程具有广阔的前景。许多深层共晶溶剂(DES)具有吸湿性,可用于水合程度不同的应用中,因此敦促深刻理解DES纳米结构随水含量的变化。在这里,我们报告了典型的氯化胆碱-尿素混合物的分子动力学模拟,使用了带参量电荷的新参数化力场来说明水合DES混合物的物理性质。这些模拟表明,即使在非常低的水合作用下,水也会改变溶液的纳米结构。我们提出了一种新颖的方法,该方法使用凸约束分析将径向分布函数分解为代表不同局部关联模式的基础组件。具体而言,DES混合物可以局部解卷积为两个主要的竞争纳米结构,其相对流行度(但不显着的结构特征)随添加的水在很宽的浓度范围内变化,从干燥到水合〜30 wt%。发现水与几种DES组分密切相关,但也明显与氯化物形成线性串珠状簇。在高含水量(超过水的约50 wt%)下,溶液变成电解质状的含水混合物。最后,DES宏观材料特性也呼应了纳米级溶液在水合作用下的结构演变。
更新日期:2020-03-30
中文翻译:

用水重构深的共晶溶剂:水合氯化胆碱/尿素的纳米结构。
深共晶混合物是有前途的,可持续的,多样化的可调溶剂类别,对各种绿色化学和工艺过程具有广阔的前景。许多深层共晶溶剂(DES)具有吸湿性,可用于水合程度不同的应用中,因此敦促深刻理解DES纳米结构随水含量的变化。在这里,我们报告了典型的氯化胆碱-尿素混合物的分子动力学模拟,使用了带参量电荷的新参数化力场来说明水合DES混合物的物理性质。这些模拟表明,即使在非常低的水合作用下,水也会改变溶液的纳米结构。我们提出了一种新颖的方法,该方法使用凸约束分析将径向分布函数分解为代表不同局部关联模式的基础组件。具体而言,DES混合物可以局部解卷积为两个主要的竞争纳米结构,其相对流行度(但不显着的结构特征)随添加的水在很宽的浓度范围内变化,从干燥到水合〜30 wt%。发现水与几种DES组分密切相关,但也明显与氯化物形成线性串珠状簇。在高含水量(超过水的约50 wt%)下,溶液变成电解质状的含水混合物。最后,DES宏观材料特性也呼应了纳米级溶液在水合作用下的结构演变。































 京公网安备 11010802027423号
京公网安备 11010802027423号