当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption Properties and Microscopic Mechanism of CO2 Capture in 1,1-Dimethyl-1,2-ethylenediamine-Grafted Metal-Organic Frameworks.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-04-13 , DOI: 10.1021/acsami.0c01927 Hui Zhang 1 , Li-Ming Yang 1 , Eric Ganz 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-04-13 , DOI: 10.1021/acsami.0c01927 Hui Zhang 1 , Li-Ming Yang 1 , Eric Ganz 2
Affiliation
|
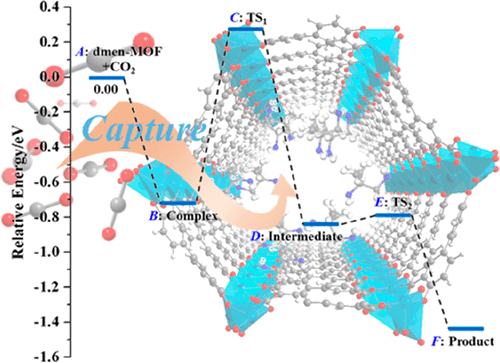
The adsorption properties and microscopic mechanism of CO2 adsorption in 1,1-dimethyl-1,2-ethylenediamine (dmen) functionalized M2(dobpdc) (dobpdc4-=4,4'-dioxidobiphenyl-3,3'-dicarboxylate; M = Mg, Sc-Zn) have been completely unveiled for the first time via comprehensive investigations based on first-principles density functional theory (DFT) calculations. The results show that for the primary-primary amine, dmen prefers to interact with the open metal site of M2(dobpdc) via the end with smaller steric hindrance. The binding energies of dmen with MOFs are in the range of 104-174 kJ/mol. In presence of CO2, it fully inserts into the metal-N bond, forming ammonium carbamate. The CO2 binding energies vary from 53 to 89 kJ/mol, showing strong metal dependence. Among the 11 metals, dmen-Sc2(dobpdc) and dmen-Mg2(dobpdc) have the highest CO2 binding energies of 89 and 84 kJ/mol, respectively, and may have large CO2 adsorption capacity for practical applications. More importantly, the microscopic CO2 capture process of dmen-M2(dobpdc) is revealed at the atomic level. The whole reaction process includes two steps, that is, formation of zwitterion intermediate (step 1) and rearrangement of the zwitterion intermediate (step 2). The first step in which nucleophilic addition between CO2 and the metal-bound amine and proton transfer from the metal-bound amine to free amine simultaneously occur is a rate-determining step, with higher energy barriers (0.99-1.35 eV). The second step with much lower barriers (maximum of 0.16 eV) is extremely easy, which can promote the whole CO2 uptake process in dmen-M2(dobpdc). This study provides a fundamental understanding of the underlying mechanism of the rather complicated CO2 adsorption process and sheds important insights on design, synthesis, and optimization of highly efficient CO2 capture materials.
中文翻译:

1,2-二甲基-1,2-乙二胺接枝的金属-有机骨架中CO2的吸附性质和微观机理。
CO2在1,1-二甲基-1,2-乙二胺(dmen)功能化的M2(dobpdc)(dobpdc4- = 4,4'-dioxidobiphenyl-3,3'-dicarboxylate; M = Mg)中的吸附性质和微观机理(Sc-Zn),已经通过基于第一原理密度泛函理论(DFT)计算的全面研究首次完全揭晓。结果表明,对于伯伯胺,dmen倾向于通过具有较小空间位阻的末端与M2(dobpdc)的开放金属位点相互作用。dmen与MOF的结合能在104-174 kJ / mol的范围内。在存在CO2的情况下,它会完全插入金属-N键中,从而形成氨基甲酸铵。CO 2的结合能在53至89 kJ / mol之间变化,显示出强烈的金属依赖性。在11种金属中,dmen-Sc2(dobpdc)和dmen-Mg2(dobpdc)的最高CO2结合能分别为89和84 kJ / mol,在实际应用中可能具有较大的CO2吸附能力。更重要的是,在原子水平上揭示了dmen-M2(dobpdc)的微观CO2捕集过程。整个反应过程包括两个步骤,即两性离子中间体的形成(步骤1)和两性离子中间体的重排(步骤2)。CO 2与金属结合的胺之间亲核加成以及质子从金属结合的胺向游离胺的转移同时发生的第一步是速率确定步骤,具有较高的能量垒(0.99-1.35 eV)。具有低得多的势垒(最大0.16 eV)的第二步非常容易,它可以促进dmen-M2(dobpdc)中的整个CO2吸收过程。
更新日期:2020-04-23
中文翻译:

1,2-二甲基-1,2-乙二胺接枝的金属-有机骨架中CO2的吸附性质和微观机理。
CO2在1,1-二甲基-1,2-乙二胺(dmen)功能化的M2(dobpdc)(dobpdc4- = 4,4'-dioxidobiphenyl-3,3'-dicarboxylate; M = Mg)中的吸附性质和微观机理(Sc-Zn),已经通过基于第一原理密度泛函理论(DFT)计算的全面研究首次完全揭晓。结果表明,对于伯伯胺,dmen倾向于通过具有较小空间位阻的末端与M2(dobpdc)的开放金属位点相互作用。dmen与MOF的结合能在104-174 kJ / mol的范围内。在存在CO2的情况下,它会完全插入金属-N键中,从而形成氨基甲酸铵。CO 2的结合能在53至89 kJ / mol之间变化,显示出强烈的金属依赖性。在11种金属中,dmen-Sc2(dobpdc)和dmen-Mg2(dobpdc)的最高CO2结合能分别为89和84 kJ / mol,在实际应用中可能具有较大的CO2吸附能力。更重要的是,在原子水平上揭示了dmen-M2(dobpdc)的微观CO2捕集过程。整个反应过程包括两个步骤,即两性离子中间体的形成(步骤1)和两性离子中间体的重排(步骤2)。CO 2与金属结合的胺之间亲核加成以及质子从金属结合的胺向游离胺的转移同时发生的第一步是速率确定步骤,具有较高的能量垒(0.99-1.35 eV)。具有低得多的势垒(最大0.16 eV)的第二步非常容易,它可以促进dmen-M2(dobpdc)中的整个CO2吸收过程。































 京公网安备 11010802027423号
京公网安备 11010802027423号