当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Chemoselectivity in Palladium-Catalyzed Three-Component Reaction of o-Bromobenzaldehyde, N-Tosylhydrazone, and Methanol
Organic Letters ( IF 4.9 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.orglett.0c01040 Xiaojian Ren 1 , Lei Zhu 2 , Yinghua Yu 2 , Zhi-Xiang Wang 1 , Xueliang Huang 2
Organic Letters ( IF 4.9 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.orglett.0c01040 Xiaojian Ren 1 , Lei Zhu 2 , Yinghua Yu 2 , Zhi-Xiang Wang 1 , Xueliang Huang 2
Affiliation
|
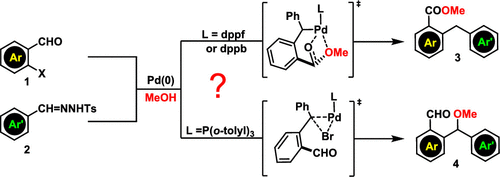
To understand the ligand-controlled palladium-catalyzed coupling of o-bromobenzaldehyde, N-tosylhydrazone, and methanol to give methyl 2-benzylbenzoic ester or methyl ether, we herein investigated the mechanisms which account for how C–C and C–O bonds are formed and why bidentate dppf/dppb ligands afford ester, whereas P(o-tolyl)3 ligand gives ether. The ester chemoselectivity of the bidentate ligands is attributed to the strong electron-donating effect that disfavors the C,Br-reductive elimination of the coupling intermediate of o-bromobenzaldehyde and N-tosylhydrazone.
中文翻译:

了解钯催化邻溴代苯甲醛,N-甲苯磺酰hydr和甲醇的三组分反应中的化学选择性
为了理解邻位-溴苯甲醛,N-甲苯磺酰hydr和甲醇的配体控制的钯催化偶联,得到甲基2-苄基苯甲酸酯或甲基醚,我们在这里研究了解释CC和C-O键如何起作用的机理。二齿的dppf / dppb配体形成酯的原因,而P(邻甲苯基)3配体生成醚的原因。二齿配体的酯化学选择性归因于强的给电子作用,该作用不利于邻溴苯甲醛和N-甲苯磺酰the的C,Br-还原消除。
更新日期:2020-04-24
中文翻译:

了解钯催化邻溴代苯甲醛,N-甲苯磺酰hydr和甲醇的三组分反应中的化学选择性
为了理解邻位-溴苯甲醛,N-甲苯磺酰hydr和甲醇的配体控制的钯催化偶联,得到甲基2-苄基苯甲酸酯或甲基醚,我们在这里研究了解释CC和C-O键如何起作用的机理。二齿的dppf / dppb配体形成酯的原因,而P(邻甲苯基)3配体生成醚的原因。二齿配体的酯化学选择性归因于强的给电子作用,该作用不利于邻溴苯甲醛和N-甲苯磺酰the的C,Br-还原消除。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号