当前位置:
X-MOL 学术
›
Nat. Cell Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
KDM4A regulates the maternal-to-zygotic transition by protecting broad H3K4me3 domains from H3K9me3 invasion in oocytes.
Nature Cell Biology ( IF 17.3 ) Pub Date : 2020-03-30 , DOI: 10.1038/s41556-020-0494-z
Aditya Sankar 1, 2, 3 , Mads Lerdrup 1, 2 , Adeel Manaf 4 , Jens Vilstrup Johansen 2 , Javier Martin Gonzalez 5 , Rehannah Borup 1 , Robert Blanshard 1 , Arne Klungland 4, 6 , Klaus Hansen 2 , Claus Yding Andersen 7 , John Arne Dahl 4 , Kristian Helin 2, 3, 8 , Eva R Hoffmann 1
Nature Cell Biology ( IF 17.3 ) Pub Date : 2020-03-30 , DOI: 10.1038/s41556-020-0494-z
Aditya Sankar 1, 2, 3 , Mads Lerdrup 1, 2 , Adeel Manaf 4 , Jens Vilstrup Johansen 2 , Javier Martin Gonzalez 5 , Rehannah Borup 1 , Robert Blanshard 1 , Arne Klungland 4, 6 , Klaus Hansen 2 , Claus Yding Andersen 7 , John Arne Dahl 4 , Kristian Helin 2, 3, 8 , Eva R Hoffmann 1
Affiliation
|
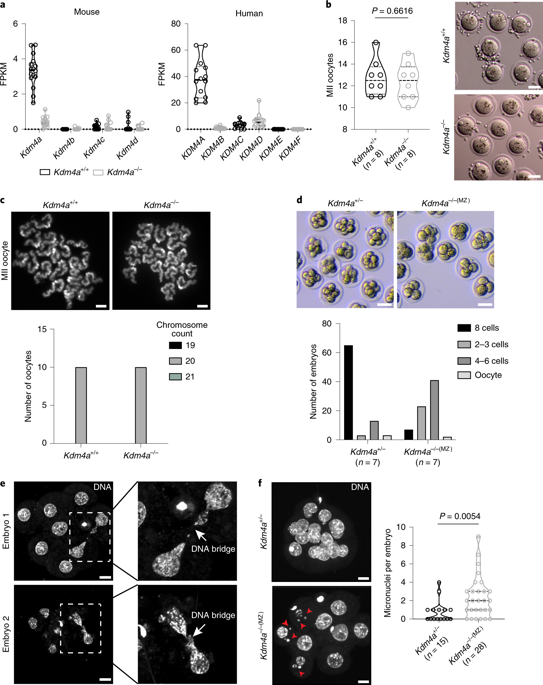
The importance of germline-inherited post-translational histone modifications on priming early mammalian development is just emerging1-4. Histone H3 lysine 9 (H3K9) trimethylation is associated with heterochromatin and gene repression during cell-fate change5, whereas histone H3 lysine 4 (H3K4) trimethylation marks active gene promoters6. Mature oocytes are transcriptionally quiescent and possess remarkably broad domains of H3K4me3 (bdH3K4me3)1,2. It is unknown which factors contribute to the maintenance of the bdH3K4me3 landscape. Lysine-specific demethylase 4A (KDM4A) demethylates H3K9me3 at promoters marked by H3K4me3 in actively transcribing somatic cells7. Here, we report that KDM4A-mediated H3K9me3 demethylation at bdH3K4me3 in oocytes is crucial for normal pre-implantation development and zygotic genome activation after fertilization. The loss of KDM4A in oocytes causes aberrant H3K9me3 spreading over bdH3K4me3, resulting in insufficient transcriptional activation of genes, endogenous retroviral elements and chimeric transcripts initiated from long terminal repeats during zygotic genome activation. The catalytic activity of KDM4A is essential for normal epigenetic reprogramming and pre-implantation development. Hence, KDM4A plays a crucial role in preserving the maternal epigenome integrity required for proper zygotic genome activation and transfer of developmental control to the embryo.
中文翻译:

KDM4A通过保护广泛的H3K4me3域免受卵母细胞中H3K9me3的侵袭,从而调节从母体到合子的转变。
种系继承的翻译后组蛋白修饰对启动早期哺乳动物发育的重要性刚刚出现1-4。组蛋白H3赖氨酸9(H3K9)三甲基化与细胞命运变化过程中的异染色质和基因阻抑有关5,而组蛋白H3赖氨酸4(H3K4)三甲基化则标记了活跃的基因启动子6。成熟的卵母细胞在转录上处于静止状态,并具有H3K4me3(bdH3K4me3)1,2的显着宽域。尚不清楚哪些因素有助于bdH3K4me3景观的维持。赖氨酸特异性脱甲基酶4A(KDM4A)在主动转录体细胞中以H3K4me3标记的启动子处使H3K9me3脱甲基。在这里,我们报道卵母细胞中bdH3K4me3处的KDM4A介导的H3K9me3去甲基化对于正常的植入前发育和受精后的合子基因组激活至关重要。卵母细胞中KDM4A的缺失导致H3K9me3在bdH3K4me3上扩散,导致基因,内源性逆转录病毒元件的转录激活不足,以及在合子基因组激活期间从长末端重复序列起始的嵌合转录物。KDM4A的催化活性对于正常的表观遗传重编程和植入前发育至关重要。因此,KDM4A在维持适当的合子基因组激活和将发育控制转移到胚胎所需的母体表观基因组完整性中起着至关重要的作用。KDM4A的催化活性对于正常的表观遗传重编程和植入前发育至关重要。因此,KDM4A在维持适当的合子基因组激活和将发育控制转移到胚胎所需的母体表观基因组完整性中起着至关重要的作用。KDM4A的催化活性对于正常的表观遗传重编程和植入前发育至关重要。因此,KDM4A在维持适当的合子基因组激活和将发育控制转移到胚胎所需的母体表观基因组完整性中起着至关重要的作用。
更新日期:2020-04-24
中文翻译:

KDM4A通过保护广泛的H3K4me3域免受卵母细胞中H3K9me3的侵袭,从而调节从母体到合子的转变。
种系继承的翻译后组蛋白修饰对启动早期哺乳动物发育的重要性刚刚出现1-4。组蛋白H3赖氨酸9(H3K9)三甲基化与细胞命运变化过程中的异染色质和基因阻抑有关5,而组蛋白H3赖氨酸4(H3K4)三甲基化则标记了活跃的基因启动子6。成熟的卵母细胞在转录上处于静止状态,并具有H3K4me3(bdH3K4me3)1,2的显着宽域。尚不清楚哪些因素有助于bdH3K4me3景观的维持。赖氨酸特异性脱甲基酶4A(KDM4A)在主动转录体细胞中以H3K4me3标记的启动子处使H3K9me3脱甲基。在这里,我们报道卵母细胞中bdH3K4me3处的KDM4A介导的H3K9me3去甲基化对于正常的植入前发育和受精后的合子基因组激活至关重要。卵母细胞中KDM4A的缺失导致H3K9me3在bdH3K4me3上扩散,导致基因,内源性逆转录病毒元件的转录激活不足,以及在合子基因组激活期间从长末端重复序列起始的嵌合转录物。KDM4A的催化活性对于正常的表观遗传重编程和植入前发育至关重要。因此,KDM4A在维持适当的合子基因组激活和将发育控制转移到胚胎所需的母体表观基因组完整性中起着至关重要的作用。KDM4A的催化活性对于正常的表观遗传重编程和植入前发育至关重要。因此,KDM4A在维持适当的合子基因组激活和将发育控制转移到胚胎所需的母体表观基因组完整性中起着至关重要的作用。KDM4A的催化活性对于正常的表观遗传重编程和植入前发育至关重要。因此,KDM4A在维持适当的合子基因组激活和将发育控制转移到胚胎所需的母体表观基因组完整性中起着至关重要的作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号