当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cuprous ion (Cu+) doping induced surface/interface engineering for enhancing the CO2 photoreduction capability of W18O49 nanowires.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.jcis.2020.03.090 Mengmeng Zhang 1 , Gang Cheng 1 , Yi Wei 1 , Zhipan Wen 1 , Rong Chen 1 , Jinyan Xiong 2 , Weijie Li 3 , Chao Han 3 , Zhen Li 4
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.jcis.2020.03.090 Mengmeng Zhang 1 , Gang Cheng 1 , Yi Wei 1 , Zhipan Wen 1 , Rong Chen 1 , Jinyan Xiong 2 , Weijie Li 3 , Chao Han 3 , Zhen Li 4
Affiliation
|
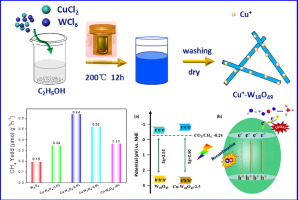
Solar-driven reduction of CO2 and H2O into fuels is a promising approach for addressing global warming and energy crisis. Herein, Cu+ doped W18O49 nanowires were prepared by a facile solvothermal method and applied in photocatalytic reduction of CO2. The composition and structure of pristine and Cu+ doped W18O49 samples have been characterized. It was found that the morphology of W18O49 nanowires was changed with increasing amounts of dopant. The photocatalytic CO2 reduction activity of W18O49 nanowires and the Cu+ doped W18O49 samples were evaluated using H2O as reducing agent. The strategy of Cu+ doping not only could affect the band edge position and the surface wettability, but also influenced separation of the photogenerated electron-hole pairs. It was found that Cu+ doping could introduce oxygen vacancy and change the conduction edge to a more negative position for W18O49 nanowires, which might be beneficial for the activation of CO2 and promote the following CO2 reduction. Furthermore, the higher separation efficiency of photogenerated electron-hole pairs with Cu+ doping could contribute to the CO2 photoreduction enhancement. In addition, the Cu+ doped W18O49 nanowires (Cu-W18O49-0.005) presented a relatively poor hydrophilic property, which might be beneficial for the adsorption of CO2 molecules and contribute to its superior photocatalytic CO2 reduction capability.
中文翻译:

铜(Cu +)掺杂诱导的表面/界面工程,用于增强W18O49纳米线的CO2光还原能力。
太阳能驱动的将CO2和H2O还原为燃料是解决全球变暖和能源危机的有前途的方法。在这里,Cu +掺杂的W18O49纳米线通过简便的溶剂热法制备,并用于光催化还原CO2。原始和Cu +掺杂的W18O49样品的组成和结构均已表征。发现W18O49纳米线的形态随着掺杂剂的增加而改变。使用H2O作为还原剂评估W18O49纳米线和掺杂Cu +的W18O49样品的光催化CO2还原活性。Cu +的掺杂策略不仅会影响能带边缘位置和表面润湿性,而且还会影响光生电子-空穴对的分离。已经发现,Cu +掺杂可以引入氧空位并将W18O49纳米线的传导边缘改变为更负的位置,这可能有益于CO2的活化并促进随后的CO2还原。此外,掺杂Cu +的光生电子-空穴对的更高分离效率可有助于增强CO 2光还原。另外,Cu +掺杂的W18O49纳米线(Cu-W18O49-0.005)具有相对较差的亲水性,这可能有利于CO2分子的吸附并有助于其优异的光催化CO2还原能力。Cu +掺杂产生的光生电子-空穴对的较高分离效率可有助于增强CO2的光还原能力。另外,Cu +掺杂的W18O49纳米线(Cu-W18O49-0.005)具有相对较差的亲水性,这可能有利于CO2分子的吸附并有助于其优异的光催化CO2还原能力。Cu +掺杂产生的光生电子-空穴对的较高分离效率可有助于增强CO2的光还原能力。另外,Cu +掺杂的W18O49纳米线(Cu-W18O49-0.005)具有相对较差的亲水性,这可能有利于CO2分子的吸附并有助于其优异的光催化CO2还原能力。
更新日期:2020-03-28
中文翻译:

铜(Cu +)掺杂诱导的表面/界面工程,用于增强W18O49纳米线的CO2光还原能力。
太阳能驱动的将CO2和H2O还原为燃料是解决全球变暖和能源危机的有前途的方法。在这里,Cu +掺杂的W18O49纳米线通过简便的溶剂热法制备,并用于光催化还原CO2。原始和Cu +掺杂的W18O49样品的组成和结构均已表征。发现W18O49纳米线的形态随着掺杂剂的增加而改变。使用H2O作为还原剂评估W18O49纳米线和掺杂Cu +的W18O49样品的光催化CO2还原活性。Cu +的掺杂策略不仅会影响能带边缘位置和表面润湿性,而且还会影响光生电子-空穴对的分离。已经发现,Cu +掺杂可以引入氧空位并将W18O49纳米线的传导边缘改变为更负的位置,这可能有益于CO2的活化并促进随后的CO2还原。此外,掺杂Cu +的光生电子-空穴对的更高分离效率可有助于增强CO 2光还原。另外,Cu +掺杂的W18O49纳米线(Cu-W18O49-0.005)具有相对较差的亲水性,这可能有利于CO2分子的吸附并有助于其优异的光催化CO2还原能力。Cu +掺杂产生的光生电子-空穴对的较高分离效率可有助于增强CO2的光还原能力。另外,Cu +掺杂的W18O49纳米线(Cu-W18O49-0.005)具有相对较差的亲水性,这可能有利于CO2分子的吸附并有助于其优异的光催化CO2还原能力。Cu +掺杂产生的光生电子-空穴对的较高分离效率可有助于增强CO2的光还原能力。另外,Cu +掺杂的W18O49纳米线(Cu-W18O49-0.005)具有相对较差的亲水性,这可能有利于CO2分子的吸附并有助于其优异的光催化CO2还原能力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号