当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of CO on Dry CH4 Oxidation on Pd/Al2O3 by Operando Spectroscopy: A Multitechnique Modulated Excitation Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-27 , DOI: 10.1021/acscatal.9b05541 Valentina Marchionni 1 , Maarten Nachtegaal 1 , Davide Ferri 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-27 , DOI: 10.1021/acscatal.9b05541 Valentina Marchionni 1 , Maarten Nachtegaal 1 , Davide Ferri 1
Affiliation
|
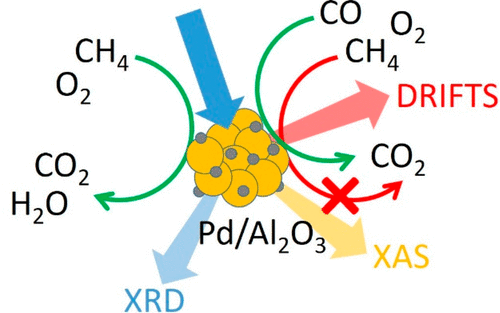
Carbon monoxide (CO) is a major pollutant present in automotive exhaust gases, including stoichiometric engines fueled by natural gas. In this work, the inhibiting role of CO on the chemistry responsible for the abatement of methane (CH4) is studied on a Pd/Al2O3 catalyst using a multitechnique approach combined with modulated excitation and mass spectrometry to obtain operando conditions. Identical modulation experiments were performed using X-ray diffraction, X-ray absorption, and diffuse reflectance infrared spectroscopy by pulsing alternately CH4 and O2 or CH4 + CO and O2 at 320 °C in the absence of steam. During the O2 pulse, reoxidation of metallic Pd was observed to occur in two steps that are associated with the initial formation of a surface layer followed by crystalline PdO. The time-resolved study showed that reduction is faster than oxidation and that it is promoted by the presence of CO. Beside determining the redox properties of the catalyst under these conditions, the presence of hydrides and Pd carbide-like species could be followed by exploiting phase-sensitive detection and its power to enhance subtle changes in the spectroscopy and diffraction data. While at the beginning of the reduction pulse CO2 was produced from reaction of CH4 with PdO, CH4 decomposition occurred on reduced Pd within the reduction pulse. In the presence of CO, CH4 oxidation at the beginning of the reduction pulse was significantly limited by reaction of CO with PdO and by blockage of the accessible Pd surface by CO adsorption.
中文翻译:

用操作光谱法研究CO对Pd / Al 2 O 3上CH 4干氧化的影响:多技术调制激发研究
一氧化碳(CO)是汽车尾气中的主要污染物,包括以天然气为燃料的化学计量发动机。在这项工作中,使用多技术方法结合调制激发和质谱技术,在Pd / Al 2 O 3催化剂上研究了CO对负责减少甲烷(CH 4)的化学的抑制作用,从而获得了操作条件。使用X射线衍射,X射线吸收和漫反射红外光谱法,通过在没有蒸汽的情况下在320°C下交替脉冲CH 4和O 2或CH 4 + CO和O 2进行相同的调制实验。在O 2期间在脉冲之后,观察到金属Pd的再氧化发生在两个步骤中,这与表面层的初始形成以及随后的结晶PdO有关。时间分辨的研究表明,还原反应比氧化反应更快,并且CO的存在促进了还原反应。除了在这些条件下确定催化剂的氧化还原性质外,还可以通过利用氢化物和类似Pd碳化物的存在来追踪相位敏感检测及其增强光谱和衍射数据微妙变化的能力。在还原脉冲开始时,由于CH 4与PdO的反应而产生了CO 2,但在还原脉冲内还原的Pd上发生了CH 4分解。在CO,CH 4存在下 还原脉冲开始时的氧化受到CO与PdO的反应以及由于CO吸附而阻塞可触及的Pd表面的明显限制。
更新日期:2020-04-23
中文翻译:

用操作光谱法研究CO对Pd / Al 2 O 3上CH 4干氧化的影响:多技术调制激发研究
一氧化碳(CO)是汽车尾气中的主要污染物,包括以天然气为燃料的化学计量发动机。在这项工作中,使用多技术方法结合调制激发和质谱技术,在Pd / Al 2 O 3催化剂上研究了CO对负责减少甲烷(CH 4)的化学的抑制作用,从而获得了操作条件。使用X射线衍射,X射线吸收和漫反射红外光谱法,通过在没有蒸汽的情况下在320°C下交替脉冲CH 4和O 2或CH 4 + CO和O 2进行相同的调制实验。在O 2期间在脉冲之后,观察到金属Pd的再氧化发生在两个步骤中,这与表面层的初始形成以及随后的结晶PdO有关。时间分辨的研究表明,还原反应比氧化反应更快,并且CO的存在促进了还原反应。除了在这些条件下确定催化剂的氧化还原性质外,还可以通过利用氢化物和类似Pd碳化物的存在来追踪相位敏感检测及其增强光谱和衍射数据微妙变化的能力。在还原脉冲开始时,由于CH 4与PdO的反应而产生了CO 2,但在还原脉冲内还原的Pd上发生了CH 4分解。在CO,CH 4存在下 还原脉冲开始时的氧化受到CO与PdO的反应以及由于CO吸附而阻塞可触及的Pd表面的明显限制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号