Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The inhibition of Kir2.1 potassium channels depolarizes spinal microglial cells, reduces their proliferation, and attenuates neuropathic pain.
Glia ( IF 5.4 ) Pub Date : 2020-03-27 , DOI: 10.1002/glia.23831 Christophe Gattlen 1, 2 , Alexandru-Florian Deftu 1, 2, 3 , Raquel Tonello 4 , Yuejuan Ling 4, 5 , Temugin Berta 4 , Violeta Ristoiu 3 , Marc René Suter 1, 2, 6
Glia ( IF 5.4 ) Pub Date : 2020-03-27 , DOI: 10.1002/glia.23831 Christophe Gattlen 1, 2 , Alexandru-Florian Deftu 1, 2, 3 , Raquel Tonello 4 , Yuejuan Ling 4, 5 , Temugin Berta 4 , Violeta Ristoiu 3 , Marc René Suter 1, 2, 6
Affiliation
|
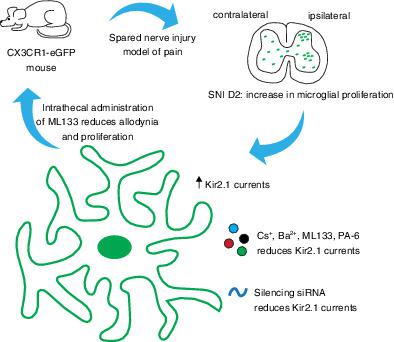
Spinal microglia change their phenotype and proliferate after nerve injury, contributing to neuropathic pain. For the first time, we have characterized the electrophysiological properties of microglia and the potential role of microglial potassium channels in the spared nerve injury (SNI) model of neuropathic pain. We observed a strong increase of inward currents restricted at 2 days after injury associated with hyperpolarization of the resting membrane potential (RMP) in microglial cells compared to later time‐points and naive animals. We identified pharmacologically and genetically the current as being mediated by Kir2.1 ion channels whose expression at the cell membrane is increased 2 days after SNI. The inhibition of Kir2.1 with ML133 and siRNA reversed the RMP hyperpolarization and strongly reduced the currents of microglial cells 2 days after SNI. These electrophysiological changes occurred coincidentally to the peak of microglial proliferation following nerve injury. In vitro, ML133 drastically reduced the proliferation of BV2 microglial cell line after both 2 and 4 days in culture. In vivo, the intrathecal injection of ML133 significantly attenuated the proliferation of microglia and neuropathic pain behaviors after nerve injury. In summary, our data implicate Kir2.1‐mediated microglial proliferation as an important therapeutic target in neuropathic pain.
中文翻译:

Kir2.1 钾通道的抑制使脊髓小胶质细胞去极化,减少其增殖,并减轻神经性疼痛。
脊髓小胶质细胞在神经损伤后改变其表型并增殖,导致神经性疼痛。我们首次对小胶质细胞的电生理特性和小胶质细胞钾通道在神经性疼痛的幸免神经损伤 (SNI) 模型中的潜在作用进行了表征。我们观察到与后期时间点和幼稚动物相比,与小胶质细胞静息膜电位 (RMP) 超极化相关的损伤后 2 天限制的内向电流强烈增加。我们在药理学和遗传学上确定电流由 Kir2.1 离子通道介导,其在 SNI 后 2 天在细胞膜上的表达增加。Kir2的抑制。含有 ML133 和 siRNA 的 1 逆转了 RMP 超极化,并在 SNI 后 2 天强烈降低了小胶质细胞的电流。这些电生理变化恰好发生在神经损伤后小胶质细胞增殖的峰值。在体外,ML133 在培养 2 天和 4 天后显着降低了 BV2 小胶质细胞系的增殖。在体内,鞘内注射 ML133 显着减弱了神经损伤后小胶质细胞的增殖和神经性疼痛行为。总之,我们的数据表明 Kir2.1 介导的小胶质细胞增殖是神经性疼痛的重要治疗靶点。ML133 在培养 2 天和 4 天后显着降低 BV2 小胶质细胞系的增殖。在体内,鞘内注射 ML133 显着减弱了神经损伤后小胶质细胞的增殖和神经性疼痛行为。总之,我们的数据表明 Kir2.1 介导的小胶质细胞增殖是神经性疼痛的重要治疗靶点。ML133 在培养 2 天和 4 天后显着降低 BV2 小胶质细胞系的增殖。在体内,鞘内注射 ML133 显着减弱了神经损伤后小胶质细胞的增殖和神经性疼痛行为。总之,我们的数据表明 Kir2.1 介导的小胶质细胞增殖是神经性疼痛的重要治疗靶点。
更新日期:2020-03-27
中文翻译:

Kir2.1 钾通道的抑制使脊髓小胶质细胞去极化,减少其增殖,并减轻神经性疼痛。
脊髓小胶质细胞在神经损伤后改变其表型并增殖,导致神经性疼痛。我们首次对小胶质细胞的电生理特性和小胶质细胞钾通道在神经性疼痛的幸免神经损伤 (SNI) 模型中的潜在作用进行了表征。我们观察到与后期时间点和幼稚动物相比,与小胶质细胞静息膜电位 (RMP) 超极化相关的损伤后 2 天限制的内向电流强烈增加。我们在药理学和遗传学上确定电流由 Kir2.1 离子通道介导,其在 SNI 后 2 天在细胞膜上的表达增加。Kir2的抑制。含有 ML133 和 siRNA 的 1 逆转了 RMP 超极化,并在 SNI 后 2 天强烈降低了小胶质细胞的电流。这些电生理变化恰好发生在神经损伤后小胶质细胞增殖的峰值。在体外,ML133 在培养 2 天和 4 天后显着降低了 BV2 小胶质细胞系的增殖。在体内,鞘内注射 ML133 显着减弱了神经损伤后小胶质细胞的增殖和神经性疼痛行为。总之,我们的数据表明 Kir2.1 介导的小胶质细胞增殖是神经性疼痛的重要治疗靶点。ML133 在培养 2 天和 4 天后显着降低 BV2 小胶质细胞系的增殖。在体内,鞘内注射 ML133 显着减弱了神经损伤后小胶质细胞的增殖和神经性疼痛行为。总之,我们的数据表明 Kir2.1 介导的小胶质细胞增殖是神经性疼痛的重要治疗靶点。ML133 在培养 2 天和 4 天后显着降低 BV2 小胶质细胞系的增殖。在体内,鞘内注射 ML133 显着减弱了神经损伤后小胶质细胞的增殖和神经性疼痛行为。总之,我们的数据表明 Kir2.1 介导的小胶质细胞增殖是神经性疼痛的重要治疗靶点。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号