当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Structurally Robust Chiral Borate Ion: Molecular Design, Synthesis, and Asymmetric Catalysis.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-27 , DOI: 10.1002/anie.202001637 Daisuke Uraguchi 1 , Fumito Ueoka 1 , Naoya Tanaka 1 , Tomohito Kizu 1 , Wakana Takahashi 1 , Takashi Ooi 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-27 , DOI: 10.1002/anie.202001637 Daisuke Uraguchi 1 , Fumito Ueoka 1 , Naoya Tanaka 1 , Tomohito Kizu 1 , Wakana Takahashi 1 , Takashi Ooi 1, 2
Affiliation
|
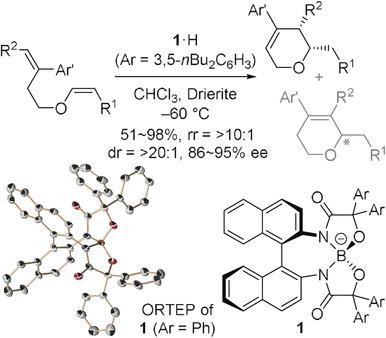
Catalysis by chiral weakly‐coordinating anions (WCAs) remains underdeveloped due to the lack of a molecular design strategy for exploiting their characteristics, such as the non‐nucleophilic nature. Here, we report the development of a chiral borate ion comprising an O,N,N,O‐tetradentate backbone, which ensures hitherto unattainable structural robustness. Upon pairing with a proton, the hydrogen borate acts as an effective catalyst for the asymmetric Prins‐type cyclization of vinyl ethers, providing access to structurally and stereochemically defined dihydropyrans. The key to selectivity control is the distinct ability of the borate ion to discriminate the prochiral faces of the acyclic oxonium ion intermediate and dictate the regiochemical outcome. We anticipate that this study paves the way for exploring the untapped potential of WCA catalysis for selective chemical synthesis.
中文翻译:

结构坚固的手性硼酸根离子:分子设计,合成和不对称催化。
手性弱配位阴离子(WCA)的催化作用仍未得到开发,原因是缺乏利用其特性(如非亲核性质)的分子设计策略。在这里,我们报告了包含O,N,N,O-四齿骨架的手性硼酸根离子的开发,这确保了迄今为止无法获得的结构坚固性。与质子配对后,硼酸氢盐可作为乙烯基醚不对称Prins型环化的有效催化剂,从而提供结构和立体化学定义的二氢吡喃。选择性控制的关键是硼酸根离子区分无环氧鎓离子中间体前手性面并决定区域化学结果的独特能力。
更新日期:2020-03-27
中文翻译:

结构坚固的手性硼酸根离子:分子设计,合成和不对称催化。
手性弱配位阴离子(WCA)的催化作用仍未得到开发,原因是缺乏利用其特性(如非亲核性质)的分子设计策略。在这里,我们报告了包含O,N,N,O-四齿骨架的手性硼酸根离子的开发,这确保了迄今为止无法获得的结构坚固性。与质子配对后,硼酸氢盐可作为乙烯基醚不对称Prins型环化的有效催化剂,从而提供结构和立体化学定义的二氢吡喃。选择性控制的关键是硼酸根离子区分无环氧鎓离子中间体前手性面并决定区域化学结果的独特能力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号