当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 8-Methyl-pyrrolo[1,2-a]pyrazin-1(2H)-one Derivatives as Highly Potent and Selective Bromodomain and Extra-Terminal (BET) Bromodomain Inhibitors.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-04-07 , DOI: 10.1021/acs.jmedchem.9b01784 Zizhou Li 1, 2 , Senhao Xiao 2, 3, 4 , Yaxi Yang 1, 2 , Chao Chen 1, 2 , Tian Lu 2, 3 , Zhifeng Chen 3 , Hualiang Jiang 3 , Shijie Chen 3 , Cheng Luo 2, 3 , Bing Zhou 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-04-07 , DOI: 10.1021/acs.jmedchem.9b01784 Zizhou Li 1, 2 , Senhao Xiao 2, 3, 4 , Yaxi Yang 1, 2 , Chao Chen 1, 2 , Tian Lu 2, 3 , Zhifeng Chen 3 , Hualiang Jiang 3 , Shijie Chen 3 , Cheng Luo 2, 3 , Bing Zhou 1, 2
Affiliation
|
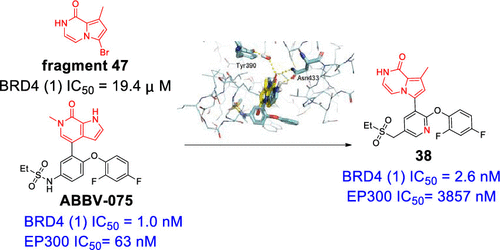
The bromodomain and extra-terminal (BET) family proteins have recently emerged as promising drug targets for cancer therapy. In this study, identification of an 8-methyl-pyrrolo[1,2-a]pyrazin-1(2H)-one fragment (47) as a new binder to the BET bromodomains and the subsequent incorporation of fragment 47 to the scaffold of ABBV-075, which recently entered Phase I clinical trials, enabled the generation of a series of highly potent BET bromodomain inhibitors. Further druggability optimization led to the discovery of compound 38 as a potential preclinical candidate. Significantly, compared with ABBV-075, which exhibits a 63-fold selectivity for BRD4(1) over EP300, compound 38 demonstrates an excellent selectivity for the BET bromodomain family over other bromodomains, with an ∼1500-fold selectivity for BRD4(1) over EP300. Orally administered 38 achieves a complete inhibition of tumor growth with a tumor growth inhibition (TGI) of 99.7% accompanied by good tolerability.
中文翻译:

发现了8-甲基-吡咯并[1,2-a]吡嗪-1(2H)-one衍生物作为高活性和选择性溴结构域和末端(BET)溴结构域抑制剂。
溴结构域和末端外(BET)家族蛋白最近已成为有希望的癌症治疗药物靶标。在这项研究中,鉴定了一个8-甲基-吡咯并[1,2-a]吡嗪-1(2H)-一个片段(47)作为BET溴结构域的新结合物,随后将片段47掺入了该载体的支架中。最近进入I期临床试验的ABBV-075使能够产生一系列高效的BET溴结构域抑制剂。进一步的可药物性优化导致发现化合物38作为潜在的临床前候选药物。值得注意的是,与ABBV-075相比,BBB4(1)对BRD4(1)的选择性是EP300的63倍,化合物38对BET溴结构域家族的选择性比其他溴结构域好,而BRD4(1)的选择性约为1500倍。超过EP300。
更新日期:2020-04-24
中文翻译:

发现了8-甲基-吡咯并[1,2-a]吡嗪-1(2H)-one衍生物作为高活性和选择性溴结构域和末端(BET)溴结构域抑制剂。
溴结构域和末端外(BET)家族蛋白最近已成为有希望的癌症治疗药物靶标。在这项研究中,鉴定了一个8-甲基-吡咯并[1,2-a]吡嗪-1(2H)-一个片段(47)作为BET溴结构域的新结合物,随后将片段47掺入了该载体的支架中。最近进入I期临床试验的ABBV-075使能够产生一系列高效的BET溴结构域抑制剂。进一步的可药物性优化导致发现化合物38作为潜在的临床前候选药物。值得注意的是,与ABBV-075相比,BBB4(1)对BRD4(1)的选择性是EP300的63倍,化合物38对BET溴结构域家族的选择性比其他溴结构域好,而BRD4(1)的选择性约为1500倍。超过EP300。































 京公网安备 11010802027423号
京公网安备 11010802027423号