当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent Effects on the Symmetric and Asymmetric SN2 Reactions in the Acetonitrile Solution: A Reaction Density Functional Theory Study
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-04-03 , DOI: 10.1021/acs.jpcb.0c00607 Weiqiang Tang 1 , Jihao Zhao 1 , Peng Jiang 1, 2 , Xiaofei Xu 1 , Shuangliang Zhao 1, 3 , Zhangfa Tong 3
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-04-03 , DOI: 10.1021/acs.jpcb.0c00607 Weiqiang Tang 1 , Jihao Zhao 1 , Peng Jiang 1, 2 , Xiaofei Xu 1 , Shuangliang Zhao 1, 3 , Zhangfa Tong 3
Affiliation
|
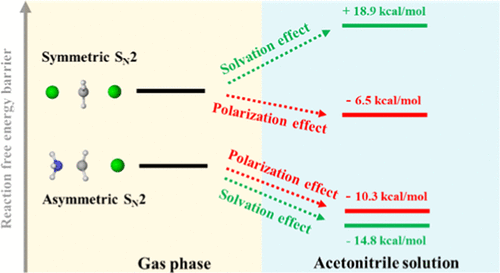
Bimolecular nucleophilic substitution (SN2) reactions are of great importance in chemistry and biochemistry due to their capability of constructing functional groups. In this work, we investigate the solvent effect on the free energy profiles of symmetric and asymmetric SN2 reactions in the acetonitrile solution using the proposed reaction density functional theory (RxDFT) method. This multiscale method utilizes quantum density functional theory for calculating intrinsic reaction free energy coupled with classical density functional theory for addressing solvation contribution. We find that the presence of acetonitrile brings both the polarization effect and solvation effect on the reaction pathways. For the eight selected symmetric SN2 reactions, the predicated reaction pathways agree well with the results from the direct and thermodynamic cycle (TC) methods with the SMD-M062X solvation model. In addition, the polarization effect reduces the free energy barriers by about 6 kcal/mol, while the solvation effect increases the barriers by about 18 kcal/mol. For the four selected asymmetric SN2 reactions, the predicted reaction pathways agree well with the results from the Monte Carlo simulations and experiments. The polarization effect and the solvation effect mutually reduce the free energy barriers, and the solvation effect plays a dominant role.
中文翻译:

溶剂对乙腈溶液中对称和不对称S N 2反应的影响:反应密度泛函理论研究
双分子亲核取代(S N 2)反应由于其能够构建官能团的能力而在化学和生物化学中具有重要意义。在这项工作中,我们使用提出的反应密度泛函理论(RxDFT)方法研究溶剂对乙腈溶液中对称和不对称S N 2反应自由能谱的影响。这种多尺度方法利用量子密度泛函理论来计算固有反应自由能,并结合经典的密度泛函理论来解决溶剂化作用。我们发现乙腈的存在给反应途径带来了极化作用和溶剂化作用。对于选择的八个对称S N在2个反应中,预测的反应路径与SMD-M062X溶剂化模型的直接和热力学循环(TC)方法的结果非常吻合。另外,极化效应使自由能垒降低了约6kcal / mol,而溶剂化效应使自由能垒提高了约18kcal / mol。对于四个选择的不对称S N 2反应,预测的反应路径与蒙特卡罗模拟和实验的结果吻合良好。极化作用和溶剂化作用相互减少了自由能垒,而溶剂化作用起主要作用。
更新日期:2020-04-06
中文翻译:

溶剂对乙腈溶液中对称和不对称S N 2反应的影响:反应密度泛函理论研究
双分子亲核取代(S N 2)反应由于其能够构建官能团的能力而在化学和生物化学中具有重要意义。在这项工作中,我们使用提出的反应密度泛函理论(RxDFT)方法研究溶剂对乙腈溶液中对称和不对称S N 2反应自由能谱的影响。这种多尺度方法利用量子密度泛函理论来计算固有反应自由能,并结合经典的密度泛函理论来解决溶剂化作用。我们发现乙腈的存在给反应途径带来了极化作用和溶剂化作用。对于选择的八个对称S N在2个反应中,预测的反应路径与SMD-M062X溶剂化模型的直接和热力学循环(TC)方法的结果非常吻合。另外,极化效应使自由能垒降低了约6kcal / mol,而溶剂化效应使自由能垒提高了约18kcal / mol。对于四个选择的不对称S N 2反应,预测的反应路径与蒙特卡罗模拟和实验的结果吻合良好。极化作用和溶剂化作用相互减少了自由能垒,而溶剂化作用起主要作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号