当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An aminoacylation ribozyme evolved from a natural tRNA-sensing T-box riboswitch.
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-03-23 , DOI: 10.1038/s41589-020-0500-6
Satoshi Ishida 1 , Naohiro Terasaka 1 , Takayuki Katoh 1 , Hiroaki Suga 1
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-03-23 , DOI: 10.1038/s41589-020-0500-6
Satoshi Ishida 1 , Naohiro Terasaka 1 , Takayuki Katoh 1 , Hiroaki Suga 1
Affiliation
|
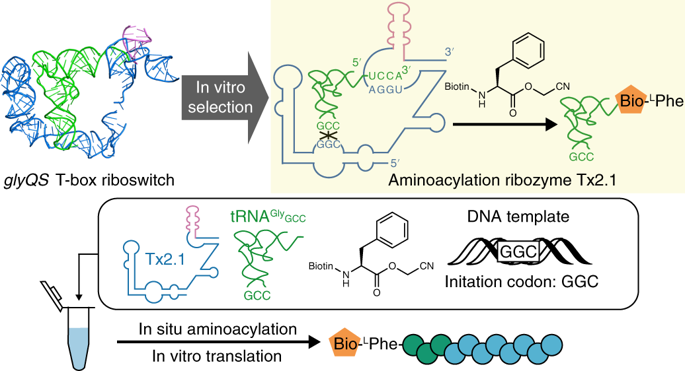
When the primitive translation system first emerged in the hypothetical RNA world, ribozymes could have been responsible for aminoacylation. Given that naturally occurring T-box riboswitches selectively sense the aminoacylation status of cognate tRNAs, we introduced a domain of random sequence into a T-box-tRNA conjugate and isolated ribozymes that were self-aminoacylating on the 3'-terminal hydroxyl group. One of them, named Tx2.1, recognizes the anticodon and D-loop of tRNA via interaction with its stem I domain, similarly to the parental T-box, and selectively charges N-biotinyl-L-phenylalanine (Bio-lPhe) onto the 3' end of the cognate tRNA in trans. We also demonstrated the ribosomal synthesis of a Bio-lPhe-initiated peptide in a Tx2.1-coupled in vitro translation system, in which Tx2.1 catalyzed specific tRNA aminoacylation in situ. This suggests that such ribozymes could have coevolved with a primitive translation system in the RNA world.
中文翻译:

氨基酰化核酶从天然的tRNA感应T盒核糖开关进化而来。
当原始翻译系统首次出现在假设的RNA世界中时,核酶可能负责氨基酰化作用。鉴于天然存在的T-box核糖开关选择性地检测相关tRNA的氨基酰化状态,我们将随机序列结构域引入T-box-tRNA结合物中,并分离出在3'-末端羟基上被自身氨基酰化的核酶。其中一个名为Tx2.1,与亲本T-box类似,通过与其茎I结构域的相互作用识别tRNA的反密码子和D环,并选择性地将N-生物素-L-苯丙氨酸(Bio-Phe)充电到反式同源tRNA的3'端。我们还证明了在Tx2.1耦合的体外翻译系统中,Bio-lPhe引发的肽的核糖体合成,其中Tx2.1催化原位特异性的tRNA氨酰化。
更新日期:2020-04-24
中文翻译:

氨基酰化核酶从天然的tRNA感应T盒核糖开关进化而来。
当原始翻译系统首次出现在假设的RNA世界中时,核酶可能负责氨基酰化作用。鉴于天然存在的T-box核糖开关选择性地检测相关tRNA的氨基酰化状态,我们将随机序列结构域引入T-box-tRNA结合物中,并分离出在3'-末端羟基上被自身氨基酰化的核酶。其中一个名为Tx2.1,与亲本T-box类似,通过与其茎I结构域的相互作用识别tRNA的反密码子和D环,并选择性地将N-生物素-L-苯丙氨酸(Bio-Phe)充电到反式同源tRNA的3'端。我们还证明了在Tx2.1耦合的体外翻译系统中,Bio-lPhe引发的肽的核糖体合成,其中Tx2.1催化原位特异性的tRNA氨酰化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号