Nature Communications ( IF 14.7 ) Pub Date : 2020-03-20 , DOI: 10.1038/s41467-020-15210-2 Alexander Riss 1 , Marcus Richter 2 , Alejandro Pérez Paz 3, 4, 5 , Xiao-Ye Wang 6, 7 , Rajesh Raju 6 , Yuanqin He 1 , Jacob Ducke 1 , Eduardo Corral 1 , Michael Wuttke 6 , Knud Seufert 1 , Manuela Garnica 1, 8 , Angel Rubio 5, 9, 10 , Johannes V Barth 1 , Akimitsu Narita 6, 11 , Klaus Müllen 6, 12 , Reinhard Berger 2 , Xinliang Feng 2 , Carlos-Andres Palma 1, 13 , Willi Auwärter 1
|
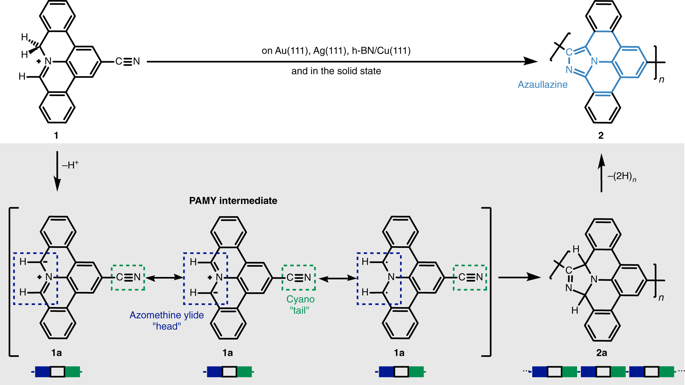
The vast potential of organic materials for electronic, optoelectronic and spintronic devices entails substantial interest in the fabrication of π-conjugated systems with tailored functionality directly at insulating interfaces. On-surface fabrication of such materials on non-metal surfaces remains to be demonstrated with high yield and selectivity. Here we present the synthesis of polyaromatic chains on metallic substrates, insulating layers, and in the solid state. Scanning probe microscopy shows the formation of azaullazine repeating units on Au(111), Ag(111), and h-BN/Cu(111), stemming from intermolecular homo-coupling via cycloaddition reactions of CN-substituted polycyclic aromatic azomethine ylide (PAMY) intermediates followed by subsequent dehydrogenation. Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry demonstrates that the reaction also takes place in the solid state in the absence of any catalyst. Such intermolecular cycloaddition reactions are promising methods for direct synthesis of regioregular polyaromatic polymers on arbitrary insulating surfaces.
中文翻译:

通过重复[3+2]环加成在金属和绝缘层上形成多环芳香链。
有机材料在电子、光电子和自旋电子器件中的巨大潜力引起了人们对直接在绝缘界面上具有定制功能的 π 共轭系统的制造产生了浓厚的兴趣。在非金属表面上制造此类材料的高产量和选择性仍有待证明。在这里,我们介绍了在金属基底、绝缘层和固态下聚芳族链的合成。扫描探针显微镜显示在 Au(111)、Ag(111) 和 h-BN/Cu(111) 上形成氮磺嗪重复单元,这是由于 CN 取代的多环芳香偶氮甲碱叶立德 (PAMY) 的环加成反应产生的分子间自偶联所致。 )中间体,然后进行脱氢。基质辅助激光解吸/电离(MALDI)质谱表明,在没有任何催化剂的情况下,该反应也在固态下发生。这种分子间环加成反应是在任意绝缘表面上直接合成立体规则性聚芳族聚合物的有前途的方法。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号